
2011 Update – Clinical trial results published open access in Cancer Immunology Immunotherapy.
“. . . Interim analysis of eight patients demonstrated significant induction of IFN-γ-producing-CD8+, -CD4+, -NK cell, and IFN-γ-producing-tumor-infiltrating-lymphocytes, signifying significant Th1 response and NK cell activation. After a median follow-up of 3.6 years, complete response (CR) + partial response (PR) = 100%, overall survival = 100%, one patient died of unrelated illness while in remission, six of seven evaluable patients are either in continuing PR/CR (5 patients) or have progression-free survival (PFS, 1 patient) exceeding the upper limit of the 95% confidence level of the genotype-specific-PFS of the phase III imatinib-monotherapy (CALGB150105/SWOGS0033), demonstrating highly promising clinical outcomes. The current trial is closed in preparation for a larger future trial. We conclude that combination of targeted therapy and immunotherapy is safe and induced significant Th1 response and NK cell activation and demonstrated highly promising clinical efficacy in GIST, thus warranting development in other tumor types.
May, 2007 – While most GIST patients experience a good initial response to Gleevec, most will eventually relapse. A new phase II GIST trial is set to try to significantly improve the initial response and duration of remission of GIST patients to Gleevec, by combining it with pegylated interferon, described later.
Nearly all of the current clinical trials for GIST involve either trying to prevent a recurrence using the current first-line treatment (Gleevec) or trying to treat the disease after it has become resistant. What has been missing is an attempt to improve upon and lengthen the excellent initial response to Gleevec. Lei Chen, M.D., Ph.D. and Professor of Medicine at the Huntsman Cancer Institute at the University of Utah and her colleagues have planned a new trial to do just that.
“The two major obstacles of durable remission in cancer patients are acquired drug-resistant clones and tumor stem cells” according to Chen. “Although GIST has initial excellent response, more than half of patients develop Gleevec resistance in less than 2 years. The responders are committed to Gleevec life-long because of the “tumor stem cell”, which will regenerate as soon as Gleevec is discontinued. GIST is a great model to prove the concept of combination targeted therapy and immunotherapy.” (Note: See the February 2005 LRG newsletter for a discussion about cancer stem cells.)
Most tumors can induce the patient’s immune system into becoming “tolerant” of the tumors and paralyze the patient’s antitumor immunity. Cytotoxic chemotherapeutic agents can cause bone marrow suppression and a low white cell count. Targeted therapy, e.g. Gleevec, does not cause significant white cell count suppression, thus preserving the patient’s immune system during treatment.
According to Dr. Chen:
“Gleevec can result in effective and rapid apoptosis and necrosis of Gleevec-sensitive cells. This rapid killing of GISTs will allow ‘restoration’ of patients’ previously paralyzed anti-tumor immunity. The massive release of tumor- specific antigens from apoptosis and necrosis can stimulate NK cells and modulate antigen- presenting cells toward development of effective adaptive anti-tumor activity if we provide help to improve the cytokine milieu and empower the endogenous anti-tumor immunity by appropriate coordination of immunotherapy with targeted therapy. Effective endogenous anti-tumor immunity can recognize and kill the Gleevec resistant clones and tumor stem cells that escaped Gleevec, the cells that eventually cause Gleevec resistance.”
In order to stimulate/optimize an immune response against GIST, the new phase II trial will add pegylated interferon α 2b (PEG-intron, Schering Plough) to Gleevec. “If given at the right dose, [with the] right timing, combined with the right drug, interferon α holds the greatest potential in breaking immune tolerance and shifting to immune stimulation against patients’ tumors,” said Chen, “with pegylated interferon α 2b we expect much improved toxicity.”
PEG-intron differs from interferon α 2b by having molecules of polyethylene glycol (PEG) attached to them. PEG causes the interferon α to remain in the body longer and prolong the effects of the interferon α and its effectiveness. It also greatly improves the tolerability and requires only once-a-week treatment.
The Trial
Dr. Chen at Huntsman Cancer Institute plans to enroll approximately thirty GIST patients for a phase II trial. The trial is expected to 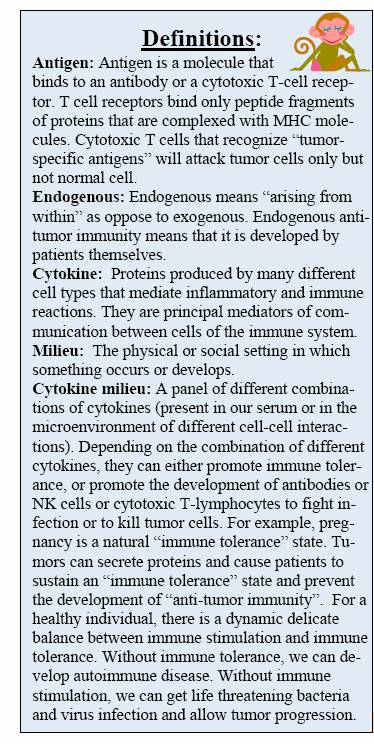 open in May 2007, initially as a single institute trial. It is expected to expand later to a multicenter trial. Because the intent is to build upon capture of the tumor-specific antigen release from Gleevec-sensitive tumors, the trial will be targeted to patients that have never received Gleevec before. In addition, GIST patients that received Gleevec as adjuvant (preventative) treatment and later developed a recurrence, are eligible only if the disease-free survival is greater than or equal to six months after completion of adjuvant Gleevec.
open in May 2007, initially as a single institute trial. It is expected to expand later to a multicenter trial. Because the intent is to build upon capture of the tumor-specific antigen release from Gleevec-sensitive tumors, the trial will be targeted to patients that have never received Gleevec before. In addition, GIST patients that received Gleevec as adjuvant (preventative) treatment and later developed a recurrence, are eligible only if the disease-free survival is greater than or equal to six months after completion of adjuvant Gleevec.
Because this trial uses immunotherapy, there are certain restrictions. Patients must have a relatively healthy immune system. They cannot have autoimmune disease or be immunosuppressed and they must have a spleen. (Note: GIST patients may have had their spleens removed during surgery.)
In addition to the attempt to improve response and remission to Gleevec, the trial has several other innovative aspects. Patients will initially receive PEG-intron plus 400 mg of Gleevec. They will then have genotyping performed and patients that have an exon 11 mutation will continue taking 400 mg. All other patients will have their Gleevec dose increased to 800 mg. In addition, if the patient has resectable disease after 24 weeks of treatment the patient will undergo surgery at week 24-25.
This trial is the first to take advantage of recent data about the mutation/dose relationship as well as an emerging trend to evaluate patients for surgery at the time of maximum response. The trial will also measure response, not only with RECIST (the current standard), but also using the “Choi criteria”. The Choi criteria seems to be one of the leading candidates to replace, or at least supplement the RECIST standard, which has proven problematic with GIST.
PEG-intron will be given at 4mcg/kg subcutaneously once per week for seven weeks. The dose will then be decreased to 1 mcg/kg weekly for 49 weeks. Like all trials, protocols are built-in to allow dose reductions for excessive toxicity.
Chen and colleagues have taken the challenge of drug-resistant clones and tumor stem cells and designed a trial combining targeted therapy and immunotherapy. They are aiming high for a long-term remission or cure and are truly offering a new and interesting opportunity to GIST patients.




