The Life Raft Group has been speaking a lot lately about plasma level testing. Many GIST survivors and caregivers have asked questions about its relevance/ importance and how to go about getting tested. In this issue, we hope to clarify this process. The following article by LRG Science Coordinator, Jerry Call will explain its relevance and discusses a presentation made by Dr. George Demetri at the 2008 Gastrointestinal Cancers Symposium.
Correlation of imatinib plasma levels in GIST patients
A higher concentration of Gleevec in the blood correlates with better clinical outcome according to George Demetri, M.D., of the Dana- Farber Cancer Institute. In an interview with Peggy Peck on the medpageTODAY website, Dr. Demetri said that the imatinib plasma level was not associated with age, gender, disease bulk, or body weight. “You really need to do pharmacokinetic testing to determine the level of imatinib because there are no clues,” Demetri reported at the Symposium. The findings suggest that “we may have been under-dosing some people,” he said.
This report is based on analysis of the pharmacokinetic data from the original phase II Gleevec trial for GIST (B2222), which started in July of 2000. Plasma levels (plasma is one
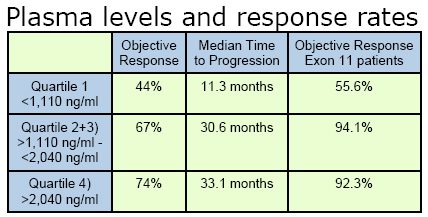 component of blood) taken after 29 days of Gleevec, were available for 73 of the 147 patients enrolled in the trial. These plasma levels were grouped into quartiles according to imatinib trough (IM) plasma concentrations (the level of drug in the blood at its lowest point during the day, just before taking the daily Gleevec capsule). The plasma levels and response rates of these groups are listed in the table above.
component of blood) taken after 29 days of Gleevec, were available for 73 of the 147 patients enrolled in the trial. These plasma levels were grouped into quartiles according to imatinib trough (IM) plasma concentrations (the level of drug in the blood at its lowest point during the day, just before taking the daily Gleevec capsule). The plasma levels and response rates of these groups are listed in the table above.
The authors concluded that, “Exposure to adequate drug levels of imatinib appears to correlate with clinical benefit; patients with the lowest imatinib levels show lowest objective response and shortest time to progression. These results suggest that monitoring pharmacokinetic/ pharmacodynamic relationships may provide novel predictive markers and that exposure to adequate IM trough plasma concentrations (>1,110 ng/mL) is important for optimal clinical response.”
A video report is available in our video section. In the interview, Dr. Demetri explained that “when you give Gleevec or any other kinase inhibitor to a group of patients, they will handle it very differently, some people will have high levels and some people will have low levels… The important part about that is whether we for years might be underdosing people, and whether we perhaps should develop a blood test to check the levels of this drug in people’s blood and have more certainty that there’s actually therapeutic levels in the blood.” Demetri went on to explain that “it’s possible that we could have done this analysis and found nothing at all, but in fact, we saw something that is a bit worrisome for the patients with the lowest levels of the drug.” The next step according to Demetri will be to “… talk with our colleagues, decide exactly how much this is worth pursuing, (and) decide how to mount a large trial.”
Testing How-To
Plasma level testing can be done by any of the doctors that you see, but remember that your doctor must register with Avantix Labs (The company that is providing the free testing) in order to do so.
Signing up:
• Complete Healthcare Provider registration form online or via fax.
• Upon approval, you will receive a unique account/User ID number via email or fax.
• Complete registration form online or via fax. Sample Collection:
• Order Sample Collection kit (The LRG handed these kits out at Life Fest, more are still available)
• You doctor should draw 5mL of blood in a purple vacutainer (with EDTA)
• Blood should be drawn no more than 2 hours before the next scheduled dose of Gleevec
• Within one hour of the collection, your doctor should centrifuge the blood sample and harvest about 2 mL of plasma and place in a 5 mL plasma polypropylene collection tube
• Place the mailing label printed from the CML Alliance website on the Test Request form before submitting sample( s) for analysis. Make sure patient and physician names are on the label
Shipping:
• Place the plasma sample with the appropriate label along with the test request form in a padded envelope. The sample may be shipped at room temperature. No dry ice or cold pack is required
• Ship the sample Monday through Thursday via FedEx overnight. Samples should not be sent on Friday or Saturday.
• Test results showing imatinib concentration will be sent to you via email or fax
• For your records, a hard copy of the result will be sent to your mailing address via U.S. Postal Service




