Plasma level testing: what we know, what we don’t & what we hope to learnThis month, Novartis Pharmaceuticals (maker of Gleevec) launched a new website designed to help optimize the outcome of GIST patients taking Gleevec. Some of the featured content is about blood level testing for GIST. In addition, the website prominently links to the Avantix labs website for testing Gleevec blood levels in CML and GIST.
The GIST Alliance site and related Avantix blood level testing site appear to be a response to the increasing awareness that testing Gleevec levels in the blood, and adjusting dose based on levels, may be superior to the current standard of assigning all patients 400 mg at the start of treatment. This position is based on preliminary data from CML and GIST trials that suggest the adequate plasma levels are critical to prevent progression.
In the GIST trial, a higher concentration of Gleevec in the blood correlates with better clinical outcome according to Dr. George Demetri, Director of the Center for Sarcoma and Bone Oncology at Dana Farber Cancer Institute. Demetri presented this information in January 2008 at the 2008 Gastrointestinal American Society of Clinical Oncology (ASCO) meeting.
 In an interview with Peggy Peck on the MedPage Today website, Dr. Demetri said that the imatinib plasma level was not associated with age, gender, disease bulk, or body weight. “You really need to do pharmacokinetic testing to determine the level of imatinib because there are no clues,” Demetri reported at the Gastrointestinal Symposium. The findings suggest that “we may have been under-dosing some people,” he said.
In an interview with Peggy Peck on the MedPage Today website, Dr. Demetri said that the imatinib plasma level was not associated with age, gender, disease bulk, or body weight. “You really need to do pharmacokinetic testing to determine the level of imatinib because there are no clues,” Demetri reported at the Gastrointestinal Symposium. The findings suggest that “we may have been under-dosing some people,” he said.
The report is based on analysis of the pharmacokinetic data from the original phase II Gleevec trial for GIST (B2222), which started in July 2000. These plasma levels were grouped into quartiles according to imatinib trough plasma concentrations (the level of drug in the blood at its lowest point during the day, just before taking that day’s Gleevec). The plasma levels and response rates of these groups are listed in the table below.
The authors concluded that, “Exposure to adequate drug levels of imatinib appears to correlate with clinical benefit; patients with the lowest imatinib levels show lowest objective response and shortest time to progression. These results suggest that monitoring PK/PD relationships may provide novel predictive markers and that exposure to adequate imatinib trough plasma concentrations (>1,110 ng/mL) is important for optimal clinical response.”
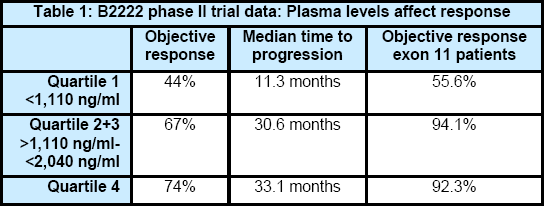 NOTE: The phase II plasma analysis divided the patients into four groups based on plasma levels (4 “quartiles”). This creates a somewhat arbitrary dose level of 1,110 ng/mL at the break point between the first and second quartile. This gives an impression that this precise number is a sort of “magic figure” (i.e., that one should feel safe to be above it, and panicked if one is below it). The data show that lower plasma levels are generally less effective, but biological common sense says that there is not a precise “safe level” for all patients. Because of this we prefer to use the approximate number of 1,100 ng/mL and will do so throughout the remainder of this article.
NOTE: The phase II plasma analysis divided the patients into four groups based on plasma levels (4 “quartiles”). This creates a somewhat arbitrary dose level of 1,110 ng/mL at the break point between the first and second quartile. This gives an impression that this precise number is a sort of “magic figure” (i.e., that one should feel safe to be above it, and panicked if one is below it). The data show that lower plasma levels are generally less effective, but biological common sense says that there is not a precise “safe level” for all patients. Because of this we prefer to use the approximate number of 1,100 ng/mL and will do so throughout the remainder of this article.
In the MedPage Today interview, Demetri went on to explain, “It’s possible that we could have done this analysis and found nothing at all, but in fact, we saw something that is a bit worrisome for the patients with the lowest levels of the drug.” According to Demetri, the next step will be to “… talk with our colleagues, decide exactly how much this is worth pursuing, [and] decide how to mount a large trial.”
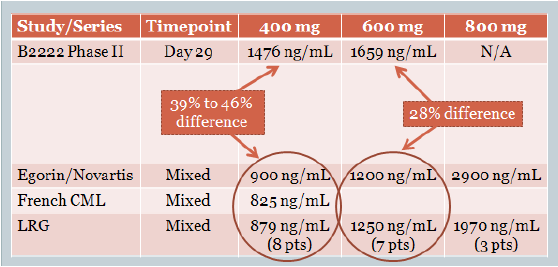
A closer look at both the B2222 trial results and other data shed light on why a trial might be needed to clarify the preliminary data that Dr. Demetri presented in 2008. In the B2222 trial, plasma levels were taken 29 days after starting Gleevec. Results were available for 73 of the 147 patients enrolled in the trial.
In addition to the fact that the patients were not randomized by plasma level, two other factors tend to confound the data. First, Professor Ian Judson and colleagues from the European Organization for Research and Treatment of Cancer (EORTC) have raised concerns that Gleevec drug levels may fall as much as 42 percent within the first year after starting Gleevec. Table 2 presents data that supports the EORTC data. This raises concerns that comparing the B2222 “Day 29” levels to plasma levels that might have been obtained at any time point may be an “apples to oranges” comparison. Still, this type of error would tend to overestimate getting an adequate amount of the drug.
Table 2: Median Plasma Levels at Different Time Points
The second major factor that confounds the B2222 plasma data is that we don’t know how many of these patients remained on the dose that they were taking at day 29. This data was not reported for the phase II trials. It may be that a significant number of patients (more likely in the 600 mg arm) had permanent dose reductions after day 29. This type of error would tend to be in the opposite direction of the first factor and tend to underestimate the actual plasma level needed to prevent progression.
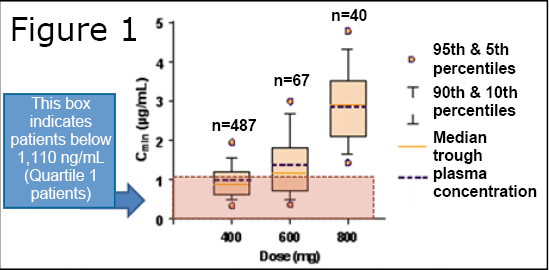 Imperfect as the plasma level data from the B2222 trial may be, it is the only plasma level/response data that we have for GIST. Additional plasma level data compiled by Novartis suggests that the majority of patients (includes CML, GIST and others) on the standard dose of Gleevec (400 mg) are below the 1,100 ng/mL level (See Figure 1).
Imperfect as the plasma level data from the B2222 trial may be, it is the only plasma level/response data that we have for GIST. Additional plasma level data compiled by Novartis suggests that the majority of patients (includes CML, GIST and others) on the standard dose of Gleevec (400 mg) are below the 1,100 ng/mL level (See Figure 1).
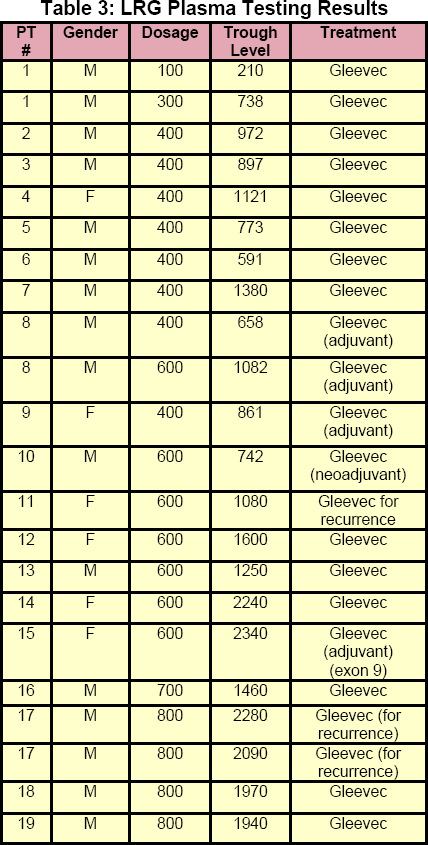
Thus far, only a small percentage of LRG members have had plasma testing and shared the results with the LRG. Those results appear in Table 3. Although only a small dataset, the results are similar to the larger dataset from Novartis. Only three of the eight patients on 400 mg had plasma levels above 1100 ng/mL. One of those patients at 1121 ng/mL had only been on Gleevec for three months and will bear close monitoring to ensure that the Gleevec level does not decline in the first year.
In the LRG data, seven patients furnished data at the 600 dose. Only one 600 mg member was clearly well below the recommended threshold with a value of 742 ng/mL. Two members were below but within three percent of the threshold with values of 1080 and 1082 ng/mL. The other four 600 mg members, one member at 700 mg and four members at 800 mg were all well above the minimum threshold.
Opinion: Will plasma testing follow
in the footsteps of mutational testing?
 Mutational data from the GIST reGISTry was recently presented at the Connective Tissue Oncology Society (CTOS) (See Jim Hughes’ CTOS article). This registry collects data on how GIST patients are being treated. One of the most striking omissions in the treatment of GIST patients is that only one percent of patients treated at local institutions are getting mutational testing. At academic institutions this number rises to four percent and even with Life Raft Group patients we only have about 20 percent of members that have reported their mutational status to us.
Mutational data from the GIST reGISTry was recently presented at the Connective Tissue Oncology Society (CTOS) (See Jim Hughes’ CTOS article). This registry collects data on how GIST patients are being treated. One of the most striking omissions in the treatment of GIST patients is that only one percent of patients treated at local institutions are getting mutational testing. At academic institutions this number rises to four percent and even with Life Raft Group patients we only have about 20 percent of members that have reported their mutational status to us.
There is a tremendous amount of data detailing the value and potential uses of mutational data. It is now “strongly encouraged” for metastatic disease and “can be considered” for patients with primary disease according to the Journal of the National Comprehensive Cancer Network (JNCCN) guidelines for GIST (July 2007). In spite of this fact, the LRG regularly hears from members that want to have the test done and are refused by their doctors. With plasma testing, refusals are even more common. Because of this, some patients have turned to their primary care doctor to order the test.
Just as disconcerting as a lack of mutational testing discussion, there were no formal discussions (that we are aware of) about plasma testing at this year’s CTOS meeting. The preliminary data suggest that plasma level testing has the potential to significantly alter the course of GIST in many patients. In this preliminary patient group, patients with levels above 1100 ng/mL had three times the duration of response as those below that level. In addition, the current practice of dosing at 400 mg may result in over half of those patients being below 1100 ng/mL as their levels decline over time.
At this time, plasma level testing may be the biggest potential opportunity to significantly improve GIST patient survival in the near future. Second-line treatment to overcome Gleevec resistance is just not where we want it to be yet.
The question remains: Will plasma testing go the way of mutational testing, with only a lucky few being offered the test? The only way this is likely to be avoided is to have a new trial designed to answer the questions that remain about plasma testing. Such a trial could probably do more and should really be designed to take advantage of all we have learned over the last eight years of Gleevec and may answer the question: What is the best way to treat patients initially?
We need to take the information that we have already learned, but are not acting on and create a first-line optimization trial with different therapies for different mutations and with the dose modified based on plasma levels for exon 11 patients. Only then will mutational testing and plasma testing move into mainstream clinical practice.
In the meantime, patients and their doctors must act on what we know today. With a median Gleevec-sensitive window of two years and no trials underway which include plasma level testing, data from future trials will be too late for most patients that are taking Gleevec right now.
You can read an editorial by Norman Scherzer on routine plasma testing in the September 2008 newsletter and a plasma testing how-to in the December 2008 newsletter.