Only six percent of GIST patients in the United States take advantage of testing that could be used to individualize their treatment according to a new article in the Annals of Oncology. Dr. Peter Pisters of MD Anderson Cancer Center in Houston, Texas and his colleagues reported results of the GIST reGISTry, a Novartis Pharmaceuticals-supported registry of 882 GIST patients in the United States.
According to the study authors, “This study pointed out several differences between clinical practice and clinical practice guidelines such as those by National Comprehensive Cancer Network (NCCN)1 and the European Society of Medical Oncology (ESMO).2 Although the majority of patients had GIST as the original diagnosis, there was poor utilization (6%) of KIT mutation testing of tumors despite KIT mutation testing being a standard practice at several academic centers, as well as recommended by both NCCN and ESMO. The authors speculated that the lack of mutational testing “…May be due to limited access to centers where testing is carried out, a lack of knowledge of the benefits of the testing, or possible differences in approach to patients with GIST treated with imatinib.” In the USA reGISTry, only one percent of patients being treated at community-based practices had mutational testing compared to 12 percent of patients at academic centers (6% combined).
 The Life Raft Group also maintains a registry of over 1,300 LRG members that are GIST patients from around the world, although the majority are from the United States. This month we will be sharing some of the data concerning mutational testing from the LRG GIST Patient Registry.
The Life Raft Group also maintains a registry of over 1,300 LRG members that are GIST patients from around the world, although the majority are from the United States. This month we will be sharing some of the data concerning mutational testing from the LRG GIST Patient Registry.
As of May 17, 2011, the LRG registry had 1,327 GIST patients; 377 (28%) of them have reported mutational testing results to the LRG. This percentage is considerably above that reported in the USA reGISTry and probably indicates a patient membership that is more actively involved in their treatment plan. It is also likely that many LRG members may actively consult and/or be treated at GIST referral centers and these centers are more likely to order mutational testing.
An interesting preliminary observation from the LRG registry noted that overall survival times for patients that had mutational testing is longer than the survival time for those that have not had testing (See Figure 1, October 2010). These survival times are calculated from the date of diagnosis.
While this observation is interesting, it doesn’t have much context. There are many reasons that aren’t related to mutational testing that these patients might have longer survival times. For example, patients with less advanced disease, that are considering adjuvant treatment with Gleevec, might get tested more often than patients with advanced disease. Examples like this, which cause the results to be hard to interpret, are called confounding factors, and there are some known and unknown confounding factors that might affect these survival results. We have examined some of the potential confounding factors that we are aware of and present some of the results in this edition of the newsletter.
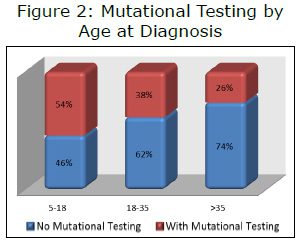
Unpublished LRG registry data has verified that, in general, younger patients (under age 35 at the time of diagnosis) have better overall survival than older patients. This is likely to be due to a relatively high percentage of patients with pediatric-type GIST in this age group. Patients with pediatric-type GIST tend to have longer overall survival than patients with adult GIST. In the LRG registry, younger patients reported having mutational testing more frequently than older patients (see Figure 2). To examine this potential confounding factor, we ran an analysis looking at only patients over age 35 at the time of diagnosis. To further enrich the population, we limited the results to only those with metastatic GIST. The results (Figure 3) were very similar to results including all patients (hazard ratio 0.55 for all patients vs. 0.53 for those diagnosed over age 35).
The LRG registry contains data on patients with both metastatic GIST and those with no metastases (“mets”). One possibility is that 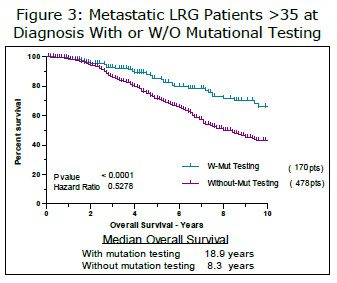 patients that are having mutational testing have less advanced disease. For example, a doctor considering offering his/her patient adjuvant Gleevec might decide to do a mutational test to verify that the patient was likely to respond to Gleevec. This patient would be likely to have a longer survival time than a patient that already had metastatic disease and the increased survival time would have nothing to do with having a mutational test. This confounding factor represents one of the most likely explanations for the differences observed and we explored this possibility from several different directions.
patients that are having mutational testing have less advanced disease. For example, a doctor considering offering his/her patient adjuvant Gleevec might decide to do a mutational test to verify that the patient was likely to respond to Gleevec. This patient would be likely to have a longer survival time than a patient that already had metastatic disease and the increased survival time would have nothing to do with having a mutational test. This confounding factor represents one of the most likely explanations for the differences observed and we explored this possibility from several different directions.
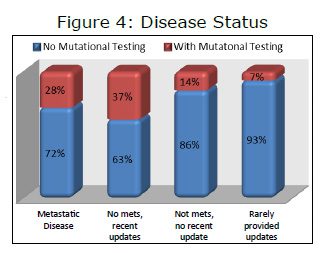
First we classified patients according to their disease stage. One category was patients that had reported metastases at any time. The second category was patients that had not reported metastases and that regularly and recently reported their status. A third category was patients that had not reported metastases but had not provided a recent update. The last category (15 patients) had seldom, if ever, provided an update beyond initial registration. At first glance this grouping appears to suggest significant differences, but when the two “no mets” groups with and without a recent update are combined and compared to the “mets” group, the difference in patients with mutational testing is small, 26 percent for no mets vs. 28 percent for mets. So the results of this analysis were not conclusive, or at best, hard to interpret. Patients with mutational testing had a median date of diagnosis of March 22, 2005 compared to a median date of diagnosis of September 9, 2003 for patients without mutational testing.
 Another way to look at the effect of disease stage was to only look at patients that already had metastatic disease when they were diagnosed. Looking at this group has several advantages. All of these patients are at a similar stage and since they all had metastatic GIST at diagnosis, the confounding factor of adjuvant treatment is eliminated. We compared the survival of 283 members (October 2010) that fit this category. Members without mutational testing had a median overall survival time of 6.3 years. The overall survival time of members with mutational testing was better but could not be defined (p value=0.0071, HR 1.83).
Another way to look at the effect of disease stage was to only look at patients that already had metastatic disease when they were diagnosed. Looking at this group has several advantages. All of these patients are at a similar stage and since they all had metastatic GIST at diagnosis, the confounding factor of adjuvant treatment is eliminated. We compared the survival of 283 members (October 2010) that fit this category. Members without mutational testing had a median overall survival time of 6.3 years. The overall survival time of members with mutational testing was better but could not be defined (p value=0.0071, HR 1.83).
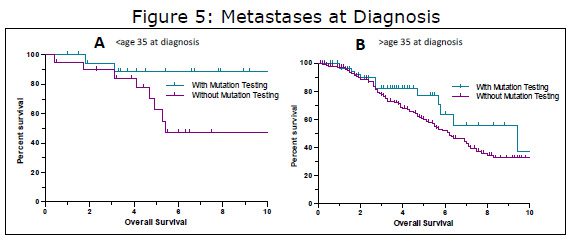
Drilling down a bit deeper into this group, we further divided the group into those diagnosed below age 35 and over 35. When doing this, both groups continued to show a benefit for mutational testing, but the differences were no longer statistical significance, possibly due to smaller sample sizes. When examining the younger group in more detail (Figure 5A), we find an example of a how a confounding factor might influence results. If we just look at mutational testing, there seems to be a fairly large difference (HR 3.25, median survival of 5.4 years vs. undefined), bordering on statistical significance (P=0.0635). But when we look at the makeup of these groups we find that the group with mutational testing has a higher percentage of females (n=17) compared to males (n=3). In the no mutation testing group, females still outnumber males, but by a smaller margin (14 females; 6 males). Females make up the vast majority of patients with pediatric-type GIST (~85%) and pediatric-type GIST is diagnosed up to age 35 and beyond (unpublished LRG data and others). This is further demonstrated when looking at the actual mutations in this group, 15 wildtype GISTs and three SDH mutations, both typical of pediatric-type GIST versus only two with KIT mutations typical of adult GIST. Thus in this group, the reason for the longer survival in those with mutation testing may not be related to how the test was interpreted, it may be because this group had more of the “good prognosis” pediatric-type GIST patients.
The mets at diagnosis, over age 35 group (Figure 5B) was a bit different however. The group with mutational testing (n=58) appeared to do somewhat better than those without testing (n=185), although the results did not quite reach statistical significance. The median survival of those with mutational testing was 9.4 years versus 6.2 years for those without mutational testing (HR 1.53, P=0.099). As yet, we have not found a confounding factor to explain the difference (and many would say that because the difference was not statistically significant, there is no difference) and we could speculate that there might be a treatment related difference. Perhaps the doctors of these patients are making mutation dependent treatment decisions. Another possibility is that these patients are very proactive (hence the mutation test) and they consult with or are treated at GIST referral centers where their treatment might be more individualized than in a community setting. Although much work remains to be done in analyzing these results, we know that there are a number of concrete reasons for doing mutational testing today. Mutational testing can be used not only to optimize current treatment, but just as importantly, it can be used to help decide which patients do not need drug treatment, as well as match patients for appropriate trials and clinics in some cases.
How can mutational testing be used?
Dose selection
The right dose in the right patient
Clinical trial results have indicated that GIST patients with KIT exon 9 tumors (about 10-13% of all GISTs) have a much longer progression-free survival time (19 months at 800 mg vs. 6 months at 400 mg) and better response to Gleevec at 800 mg compared to 400 mg. The 2010 NCCN Guidelines says this in reference to metastatic exon 9 dosing of Gleevec, “Patients with documented mutations in KIT exon 9 may benefit from dose escalation up to 800 mg daily (400 mg twice daily) depending upon tolerance”.
Patients with other mutations are given standard dose imatinib (400 mg); however, there is evidence that maintaining Gleevec plasma levels above 1100 ng/mL may significantly increase progression- free survival time for metastatic patients. This hypothesis is best supported for patients with KIT exon 11 mutations, the most common mutation occurring in GIST.3
Adjuvant Treatment
As one factor in deciding on adjuvant Gleevec
Results of the phase III Z9001 adjuvant Gleevec trial have showed significant delays in recurrences when patients are given 400 mg of Gleevec for one year. However, not all mutation subtypes have shown a benefit. In fact, of the four most common subtypes of GIST only KIT exon 11 mutations have shown a significant benefit (reported at the 2010 conference of the American Society of Clinical Oncologists (ASCO). PDGFRA D842V mutations are insensitive to Gleevec and Sutent and seem to have a lower recurrence rate that is not reduced by taking adjuvant Gleevec. To date, there has been no significant difference in recurrence for wildtype GIST either. For KIT exon 9 mutations, there appears to be an initial benefit, but the recurrence- free survival curves later crossover showing more late benefit for placebo, raising questions about proper dose and duration of treatment. At best, more information is needed for KIT exon 9 mutations. We will soon have more information about adjuvant Gleevec as final results of the Scandinavian study comparing one year of adjuvant Gleevec versus three years of adjuvant Gleevec will be presented at the plenary session of the American Society of Clinical Oncologists (ASCO) in June.
Clinical Trials and Mutation-Specific Clinics
New clinical trials for D842V (open) and wildtype GIST (planned)
A new phase II clinical trial (NCT01243346) for a specific inhibitor of the D842V mutation in PDGFRA is open for recruitment for advanced D842V GIST patients in the United States. The D842V mutation has been the most difficult mutation to target in GIST and makes up about five percent of all GISTs. This is the first trial for a specific mutation in GIST and the first drug therapy option for these patients. Ironically, most patients with this mutation will never be tested and thus never know they are eligible for this trial if current trends in mutational testing continue.
Clinical trials are also being planned for patients with wildtype GIST. Specifically, a trial with a drug that targets the IGF1R receptor is being planned. In addition, patients with wildtype GIST are eligible to attend the Wildtype & Pediatric GIST clinic hosted twice a year by the National Institutes of Health (NIH). This clinic has yielded valuable information about wildtype and pediatric GIST, most notably that pediatric-type GIST appears to be driven by mutations in the SDH complex or inactivation of SDHB, a tumor suppressor gene. Although not a clinical trial, the clinic has also published a poster of pediatric/wildtype response to various kinase inhibitors.
In summary, the United States is falling far behind other countries in terms of mutational testing for GIST patients. This is in spite of guidelines recommending testing, availability of testing through a growing network of labs, and numerous reasons for doing the testing. Mutational testing represents one of the best opportunities to individualize patient treatment. It has the potential to guide dose selection, influence the decision to take or not take adjuvant imatinib, guide patients to specific trials and help refer wildtype and pediatric-type GIST patients to additional resources. In the near future the Life Raft Group will be launching a survey to help us understand why mutational testing is seldom performed in the United States.
References
1. NCCN Task Force Report: Update on the Management of Patients with Gastrointestinal Stromal Tumors [http://www.jnccn.org/content/8/ Suppl_2/S-1.full.pdf+html?frame=header].
2. Casali PG, Blay J-Y, On behalf of the ESMO/ CONTICANET/EUROBONET Consensus Panel of Experts: Gastrointestinal stromal tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology 2010, 21:v98-v102.
3. Demetri GD, Wang Y, Wehrle E, Racine A, Nikolova Z, Blanke CD, Joensuu H, von Mehren M: Imatinib Plasma Levels Are Correlated With Clinical Benefit in Patients With Unresectable/Metastatic Gastrointestinal Stromal Tumors. Journal of Clinical Oncology 2009, 27:3141 -3147.