What Can SideEQ Do for You?
What is personalized side effect management, and why is it important for your survival and quality of life?
“Never judge a person until you’ve walked a mile in their shoes.”
As a child, I remember hearing that quote quite often. Looking at it as an adult I can appreciate that this is really good advice. It teaches us to look at things from the perspective of others. But why shoes? What do shoes have to do with someone else’s perspective? And what does this have to do with side effects? Let me explain a few things, and I believe the path will reveal itself.
Shoes and oncology
 If you have ever shopped for shoes, you know there are a myriad of choices. Shoes are made in different materials, such as leather and canvas, and come in an endless variety of styles, like dress shoes, sneakers, or even shoes designed for specific pursuits; hiking, golf, or water shoes for the surf at the beach. Moreover, shoes come in different sizes and widths, and are usually sold in women’s and men’s versions – and for at least the last few hundred years, have a left and a right one that are shaped a little bit differently to accommodate the shape of each foot. Lastly, you know that no matter how much you examine these choices, you have to actually try the shoes on, because even though two pairs may be of the same size and width, they may also fit each person very differently.
If you have ever shopped for shoes, you know there are a myriad of choices. Shoes are made in different materials, such as leather and canvas, and come in an endless variety of styles, like dress shoes, sneakers, or even shoes designed for specific pursuits; hiking, golf, or water shoes for the surf at the beach. Moreover, shoes come in different sizes and widths, and are usually sold in women’s and men’s versions – and for at least the last few hundred years, have a left and a right one that are shaped a little bit differently to accommodate the shape of each foot. Lastly, you know that no matter how much you examine these choices, you have to actually try the shoes on, because even though two pairs may be of the same size and width, they may also fit each person very differently.
The shoe salesman will help you find the shoes that fit your feet. In summary, shoes are different, because people’s feet are different. Going back to our earlier quote above, this is why you would gain a new perspective from walking in someone else’s shoes – because their feet, as their mindset, are different than yours. In effect, their shoes are “personalized” to their unique feet.
Precision oncology for a specific disease
If you have paid attention to the cancer medication landscape for the last few years, you will have begun to understand the parallel with shoes. A shoe salesman (or manufacturer) needs to take into account the individual nuances of a customer’s feet as well as their style preferences and the specific purpose the customer has in mind. To borrow a phrase from oncology, think of this not just as fitting a foot, but as engaging in “precision footwear.” Oncologists (and drug manufacturers) do something similar. They take into account the patient’s disease, tumor mutation result, and numerous other factors when prescribing or developing medications for cancer. This is referred to as “precision oncology.”
Imagine being told that these individual factors didn’t matter, but instead that no matter what type of cancer you have, you will get the same drug as everyone else. Equally ridiculous would be walking into a shoe store and finding only one type of shoe, in one size and width. For many people, that would be a solution that just doesn’t stand on its own two feet.
Precision oncology is definitely a welcome improvement, and the targeted therapies that have resulted will continue to prove beneficial to patients. GIST as a disease is a prominent example of this approach, but not the only one. Drugs have already been developed for numerous cancers that are mutation-specific, and in some cases, the benefit from these drugs has extended to multiple disease types that share the same mutation. A recent example is Keytruda (pembrolizumab), an immunotherapy drug that was developed for Non-Small Cell Lung Cancer and targets PD-1. This drug is now being used in other forms of cancer that harbor the PD-1 biomarker, such as Cholangiocarcinoma, Melanoma, and Hodgkin’s Lymphoma, and is also being studied in combination with other drugs for GIST.
This approach works because oncologists have recognized the simple truth that patients may have different cancers, but in some cases, their disease is similar enough mutation-wise that they can benefit from the same drug. But, while in many ways these patients are similar, they are also quite different, both across diseases and within their own disease. This impacts not only medication selection, but also side effect management. Let me give you a few examples of how this works.
Precision oncology & cancer medications
Imagine now that you are an oncologist and you are about to see two GIST patients back-to-back. Think about what you would prescribe for each patient. Here is the scenario for each one:
Patient #1: One primary tumor (stomach), high risk of recurrence
Patient #2: One primary tumor (stomach), high risk of recurrence
This oncology thing is pretty easy, right? Or is it?
Give each patient Gleevec and remember to collect their co-pay. Has some information has been left out? Let me fill in some details.
Patient #1: One primary tumor (stomach), high risk of recurrence, exon 11
Patient #2: One primary tumor (stomach), high risk of recurrence, wildtype
Ah ha! You can now see that the first patient would most likely benefit from Gleevec, and for the second, Gleevec is probably not the best option. Case closed, right? Well…
Patient #1: One primary tumor (stomach), high risk of recurrence, exon 11, 97 years of age, end-stage renal disease
Patient #2: One primary tumor (stomach), high risk of recurrence, wildtype, 26 years of age, in otherwise perfect health
As you are beginning to see, this oncology thing isn’t nearly as easy as it appears at first glance. But, as you can also see, the key to understanding the problem lies in gathering as much meaningful data as possible. Having this data may reveal factors that make the individual case much more specific than the “textbook” one.
Moving towards precision side effect management
How does this scenario dealing with medication selection relate to side effects? It relates in at least two major ways. First, we must take a basic look at not only what a side effect is, but also what the impact of having a side effect could be on a patient and their treatment.
When side effects are severe enough, they can affect a patient’s ability to continue taking their medication. In oncology research, this is known as compliance or adherence. The problem occurs when a patient either starts missing their medication enough to lower it to a dose that stops working or stops taking the medication altogether. When either happens, the medication is no longer being taken at its expected dose to treat the disease and the patient can have a worse outcome. This is true of any patient with any cancer. In order to prevent this from happening, oncologists and the rest of the patient’s medical team engage in what is known as “side effect management.” These are methods to prevent or treat the side effects in order to keep the patient on medication whenever possible, or switch to a different one if necessary.
Second, we have to ask ourselves a fundamental question. Just as patients are different (and these differences need to be taken into account when selecting a medication) is the same true for side effects? Will patients of different types be more likely to experience a certain side effect, and also experience different ones from other patients? If this is true, do patients need to have their side effect management altered based on these differences? At The LRG, we believe the answer to these questions is YES, and we are aiming to collect the data to show it. This is where our SideEQ platform comes in (and also where you can help).
SideEQ and the quest for more (& better) side effect data
If you’ve ever seen a commercial for a medication for a disease of any type, you probably remember hearing them report the side effects you might experience. You may have also wondered where that information comes from. In most cases, the data comes from the clinical trial that resulted in the medication’s approval by the Food and Drug Administration (FDA).
In a clinical trial, a side effect is usually termed an “adverse event” and patients are either assessed for them during their trial visits (for those events that require a diagnostic confirmation, like anemia) or are simply asked if they are experiencing them (for events like nausea, fatigue, or diarrhea). These events are then rated (usually on the National Cancer Institute (NCI) toxicity scale) and assigned a number based on their frequency or severity. This rating is geared more towards clinical outcomes as opposed to other factors, such as the impact on the quality of life. The event and its associated rating is then recorded. When the medication is approved, the side effect is reported in the prescribing information. If a patient reports a new side effect to their physician after the drug has been approved, the doctor is obligated to report it to the FDA. How quickly it must be reported varies based on the severity of the side effect, with more severe ones having to be reported immediately while those of lower ratings are allowed to be combined and reported in longer time frames.
Another question you might ask when watching a medication commercial, particularly if it’s one you are considering taking, is “will this side effect really happen to me?” The answer to that question is not that simple and goes to the heart of what we believe actually defines personalized side effect management. Trial data can tell you things like “24% of patients experienced fatigue” and also what the severity of the fatigue was. But were there any distinguishing traits among that 24 % of patients or the 76% who didn’t experience that side effect? The data rarely, if ever, reveals that information, and much like the medication example we used above, we feel that leaving out that data may mean we are missing essential information that can affect a patient’s quality of life.
Let’s take a similar approach as the one used in the medication example to illustrate the point further. You are back on the clock as an oncologist, and here are two of your patients, both of whom will be taking the same drug:
Patient #1: 46-year-old Asian male, with kidney disease, high blood pressure, and diabetes
Patient #2: 62-year-old Caucasian female, breast cancer survivor, with severe food allergies
Known side effects for this drug are nausea and fatigue. It may also cause allergic reactions or increased blood pressure in some individuals, though it is unclear what characteristics may cause that to happen based solely on the trial data. With this information, do you think it is possible that each patient could be at a different level of risk for each side effect? Would you rather know this information or not when trying to decide how to approach the management of side effects for these two individuals?
SideEQ’s purpose is to gather data exactly like this and to ultimately put that data in the hands of physicians so they can select appropriate treatments and better manage patient side effects. This is the type of data that is not always collected in clinical trials and has been recently termed by the industry press as either empirical data or Real World Evidence (RWE). Whatever you choose to call it, The LRG has been collecting it faithfully in our Patient Registry since 2000. An example of early RWE was a study on dosage of 169 LRG patients on the original Gleevec trial which we presented at CTOS in 2004. This type of data is not designed to replace clinical trial data, but to enhance it. To best understand the soul of the platform (or to continue with the shoe metaphor, perhaps the “sole”), it would serve us to look at the platform more closely.
Let’s take a look at The LRG’s approach to collecting RWE data
SideEQ is available for free at https://www.mysideeq.org for use on your computer. It is designed for use not only by GIST patients but also those with other cancers. We did this deliberately for two reasons. One, we thought, in addition to benefiting GIST patients, the platform could help all cancer patients in general, and two, we felt that collecting data from different diseases would allow us to compare across disease states and see if side effects varied even for the same medication.
The goal is to obtain a clearer picture of how to personalize side effect management. When registering, there are a number of different data elements we ask the user to provide in addition to an email, username, and password. We also ask information about gender, age, and country of birth, in an effort to paint a more specific demographic picture. We plan on adding more of these fields in the future to help further understand what factors cause side effects to vary in patients. We also ask information about the patient’s disease and medications.

A number of diseases and medications are at the top of the list. We have a special interest in looking at these diseases because they usually share a medication in common with GIST, or in the case of some medications, because they are some of the most frequent ones patients report taking. We have the ability to change this listing very easily, which gives us the flexibility to list not only drugs that are currently approved but also those that are still in trial such as Blueprint’s BLU-285 or Deciphera’s DCC-2618. We are also able to add drugs when requested by patients.
In addition to the common drugs and medications, choosing “select other” allows the patient (or caregiver, as they are also allowed to create an account to report on the patient’s behalf) to select from a larger list of diseases and medications.
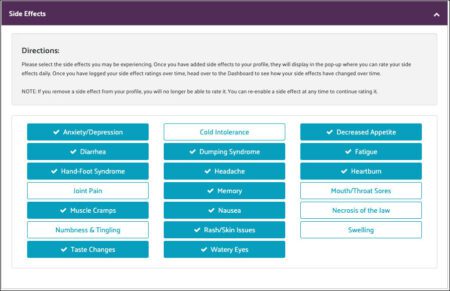
Once that is completed, the patient moves on to their newly created profile, where they can select the side effects they are currently experiencing.
This example is test data, so there may be more side effects selected than might normally be the case. Our preliminary data from SideEQ suggests that in many cases patients do report multiple side effects.
Once you have selected your side effects, you can begin rating them; this should be done periodically. We use this information to determine how side effects change over time, and you can also use it as a type of diary to see if side effect changes are associated with other life changes, such as diet or lifestyle.
The format for this rating method was selected based on our conversations with the Food and Drug Administration (FDA) when we first developed SideEQ, and its inclusion in a pilot project observational trial that the FDA suggested. One very important suggestion the FDA made was to add a question about which side effect causes a patient the most concern. This provides another level of personalization, as the side effect that most impacts a patients quality of life will vary from person to person.
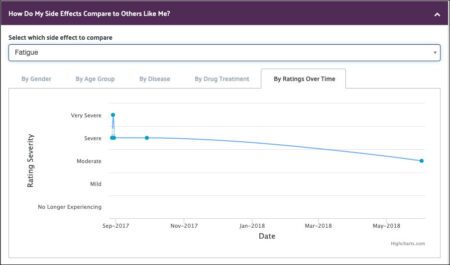
This emphasis is something not often found in standard side effect data collection and speaks to the patient’s viewpoint, which we believe is especially important when personalizing treatment.
In this example, we see the specific side effect of fatigue charted over time. As mentioned, in addition to helping The LRG understands how side effects may change over time, it also serves as a type of diary for the patient. Imagine this was your data, and you also realized that when your fatigue abated, you had also changed your diet, or perhaps started getting more sleep. Wouldn’t this be valuable information to help you manage your side effects?
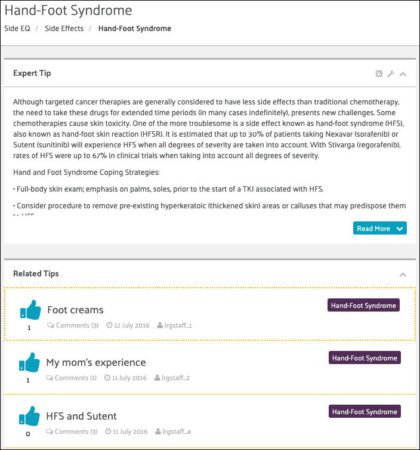
Finally, in addition to collecting data to help The LRG (and ultimately, physicians) understand over time how side effects may vary based on different factors, we want to give patients the information they can use right now to manage their side effects.
This is where the other “side” of SideEQ comes in. For each side effect, management tips for them have been provided, and there is a forum to discuss them as well as a way to post tips for management. On the left, is an example from the page on Hand-Foot Syndrome.
What does SideEQ mean for you, the patient?
We do not collect data simply for the sake of collecting. Our objective is to help patients live longer and improve their quality of life. We feel that by making side effect management more personalized, we can get closer to this goal.
What can you do to help us get closer to this goal?
If you haven’t joined SideEQ, please join us and over 400 patients who have already done so. Your data will not only help GIST (and other cancer) patients but also can help you individually, as it may allow you to see patterns that will help you better manage your side effects and improve your quality of life. This is an outcome we can all stand behind. If you already are a part of SideEQ, please continue to update your side effects and quality of life data, as we need to understand how it changes over time. Also, keep posting tips and responding to other’s posts. What we need now is more data and better data.
That can’t happen without you.




