Survey Conducted October 2001
In our April 2001 newsletter, the Life Raft Group presented its first survey of the side effects that member patients have experienced with Gleevec. This was a very early study of a small number of participants and gave us our first data on the side effects experienced by GIST patients in the Gleevec clinical trials. Member patients were asked to list up to three significant side effects and to rank each on a scale of low, medium or high. Since this first study, the number of our patient members has increased significantly and a higher dosage of 800 mg per day has been introduced with the phase lll trials. The dramatic responses of most of our members to Gleevec, combined with a growing realization that GIST is becoming a chronic disease, which may necessitate remaining on Gleevec for long periods of time, has prompted us to re-explore the issue of side effects.
In our attempt to do so, we have tried to respond to the suggestions and critiques of our earlier study. Were the side effects present prior to taking the drug? Have they changed over time? Is the ranking of severity consistent among members? Do the side effects impact upon our ability to function and, if so, how? Does weight make a difference? How are side effects being managed? How reliable is the reporting of side effects?
This Side Effects Survey builds upon our earlier study, and attempts to be more comprehensive in scope and detail. Our study group has grown to 61 member patients and now includes those on the higher 800 mg per day dose. Members are now asked to list all significant side effects and to rank them over four distinct time periods: the period prior to starting Gleevec; the first 3 months on the trial; the second 3 months on the trial; and the subsequent period on the trial (which ranged from month seven to month twelve). Building upon the work that has been done by the medical community to evaluate pain, we introduced a new scale for rating the severity of side effects. (See Appendix, Exhibit A).
For the first time, we attempt to evaluate the quality of medical management of side effects, the sources of information patients rely upon, and the issue of whether patients would minimize the reporting of their side effects in order to remain in the trials.
Objectives
The major objectives of this survey are:
- To provide patients/caregivers and physicians with better information about the type and severity of side effects that GIST patients are experiencing with Gleevec
- To evaluate the medical management of these side effects
- To empower patients/caregivers in their relationships with physicians
Caveats: We repeat our standard caveats. Although many of us come from professional backgrounds, we recognize that we are not professional researchers and our data may be subject to the twin demons of inaccuracy and distortion. Although we have continued to strengthen our survey techniques and our quality control procedures, we are cognizant that patient-based reporting may not always be accurate and that, although we have a larger study group than ever before, we do not know how representative we are of the clinical trials as a whole. Finally, we remain aware of the pitfalls of small numbers. A small change can have a disproportionate statistical effect.
The Side Effects
The survey data is based upon the reports of 61 of the 67 active member patients on board as of August 1, 2001, for a response rate of 91%. Not included were six of our seven members who have died in the past year, a number who have been removed from the trials for lack of response and a significant number of new patient members who have joined us since August 1. Included are two GIST-Gleevec patients not on the clinical trial.
Sixty (60) of the 61 respondents, or 98%, reported a total of 273 side effects for an average of 4.6 per patient. Forty-five (45) of the 61 respondents, or 74%, reported 119 severe side effects for an average of 2.6 for the 45. (See Table 1).
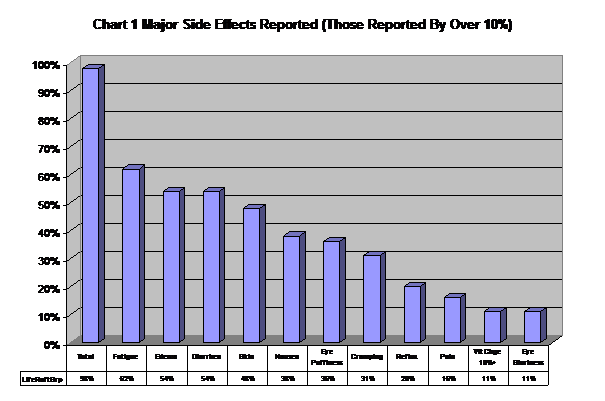
The most common side effects reported are fatigue, edema, diarrhea, skin problems, nausea, eye puffiness, cramping, reflux, pain, weight change and eye blurriness. (See Chart 1).
| Table 1 Summary Of Reported Side Effects (n = 61) | ||||||||
|
Patient Rating By Time Period |
||||||||
| Side Effect | No. Rept’d | % Rept’g | No. Severe | %SevereOf No. Reptd | PreTrial | 1stQtr | 2ndQtr | Subseq Period |
| Total | 273 | 98% | 119 | 44% | – | – | – | – |
| Fatigue |
38 |
62% |
19 |
50% |
2.7 |
5.4 |
4.8 |
4.0 |
| Edema |
33 |
54% |
21 |
64% |
0.6 |
5.4 |
4.3 |
3.1 |
| Diarrhea |
33 |
54% |
13 |
39% |
1.5 |
5.1 |
4.9 |
4.6 |
| Skin * |
29 |
48% |
13 |
39% |
0.3 |
4.1 |
4.3 |
3.7 |
| Nausea |
23 |
38% |
9 |
39% |
0.3 |
4.3 |
2.8 |
2.2 |
| Eye Puffiness |
22 |
36% |
4 |
18% |
0 |
4.0 |
3.7 |
4.1 |
| Cramping |
19 |
31% |
6 |
32% |
0.7 |
3.6 |
4.0 |
3.7 |
| Reflux |
12 |
20% |
2 |
17% |
2.2 |
4.7 |
3.4 |
4.0 |
| Pain |
10 |
16% |
5 |
50% |
2.3 |
3.6 |
3.5 |
1.8 |
| H.G.B. (Anemia) |
8 |
13% |
2 |
25% |
1.6 |
3.4 |
5.0 |
2.8 |
| Wt Chge +/-10% |
7 |
11% |
– |
– |
– |
– |
– |
– |
| Eye Blurriness |
7 |
11% |
3 |
43% |
0 |
5.0 |
4.3 |
3.0 |
| Liver Enzymes |
6 |
10% |
5 |
83% |
2.7 |
2.3 |
3.0 |
– |
| Fever |
4 |
7% |
3 |
75% |
2.0 |
5.2 |
5.2 |
4.0 |
| Mood |
4 |
7% |
1 |
25% |
0 |
1.3 |
3.0 |
2.3 |
| Hair Thinning |
4 |
7% |
0 |
0% |
0 |
2.7 |
4.0 |
4.0 |
| GI Bleed |
3 |
5% |
1 |
33% |
0 |
1.0 |
0.5 |
2.5 |
| White Bl. Cells |
2 |
3% |
2 |
100% |
3.5 |
7.5 |
3.5 |
3.54 |
| Neutrophils |
2 |
3% |
2 |
100% |
3.5 |
7.5 |
3.5 |
3.5 |
| Dizziness |
2 |
3% |
2 |
100% |
0 |
7.0 |
5.0 |
4.0 |
| Miscellaneous** |
12 |
20% |
6 |
50% |
2.2 |
5.6 |
2.2 |
3.0
|
*Skin side effects include patient reported neuropathy.
** Miscellaneous side effects include shortness of breath (2 reported), metallic taste, low blood pressure, constipation, and difficulty concentrating.
Severe Side Effects
Side effects were rated as severe if they received an average patient rating of 7 or more in any given time period or if they were cited as a reason for stopping the drug or for lowering its dosage level. The greatest number of severe side effects reported were fatigue, edema, diarrhea and skin. (See Tables 1 and 3).
Of those reporting severe side effects, 24% reported only one, 24% reported two and 52% reported three or more. (See Table 2).
|
Table 2 Number Of Severe Side Effects Reported |
||
| No. Severe SideEffects Reported | No. Respondents Reporting | % Reporting |
|
One |
11 |
24% |
|
Two |
11 |
24% |
|
Three |
11 |
24% |
|
Four |
8 |
18% |
|
Five |
3 |
7% |
|
Six |
1 |
2% |
|
Totals |
45 |
99% |
Reasons For Stopping And/Or Decreasing The Dosage Of Gleevec
Of the 61 total respondents, 6 had their drug dosages increased, 5 because of tumor growth and 1 because their side effects improved to permit increasing above an initial 100 mg/day level (this was one of the non trial participants). Of the remaining 55 respondents, 24, or 44%, reported having their drug stopped for a period of time, or their drug dosage reduced because of side effects, with edema and skin problems being the most common cause. (See Table 3).
| Table 3 Reasons For Stopping And/Or Decreasing The Dosage Of Gleevec | ||
| Number | Percent | |
| Edema |
8 |
33% |
| Skin * |
7 |
29% |
| Liver Enzymes |
3 |
13% |
| Multiple Reasons |
3 |
13% |
| Eye Blurriness |
1 |
4% |
| White Blood Cells |
1 |
4% |
| Fever |
1 |
4% |
| Totals |
24 |
100% |
* Skin side effects include patient reported neuropathy.
Functional Side Effects
We asked each respondent to separately list the functional impact that their side effects were having on their lives. Almost half report having to cope with fatigue. About one third report functional decreases in their physical activities, their sleep, their mood and their appetites. About one fifth report an impact upon their ability to work, to conduct daily activities and to concentrate. Finally, about one out of ten report an impact upon their relationships, general and sexual. (See Table 4).
| Table 4 Functional Side Effects (n = 61) | ||
| Functional Side Effect | Number Reporting | % Reporting |
| Fatigue |
30 |
49% |
| Physical Activities |
20 |
33% |
| Sleep |
19 |
31% |
| Mood |
18 |
30% |
| Appetite |
17 |
28% |
| Work |
14 |
23% |
| Daily Activities |
14 |
23% |
| Concentration |
11 |
18% |
| Sexual Relationships |
8 |
13% |
| General Relationships |
4 |
7% |
Life Style Changes
We asked each respondent to note any life style changes that they have made since starting Gleevec. Significant numbers report changes toward a healthier lifestyle, most being prompted by clinical protocol admonitions. The result, whatever the reason, is that there has been a significant decrease in the intake of caffeine and the use of alcohol. (See Table 5).
| Table 5 Life Style Changes After Starting Gleevec (n = 61) | ||
| Number | Percentage | |
| Decreased Caffeine |
28 |
46% |
| Decreased Alcohol |
26 |
43% |
| Diet Changes |
21 |
34% |
| Decreased Smoking * |
2 |
3% |
* Very few members smoked prior to the trial
Side Effects Over Time
Respondents were asked to describe and rank each of their side effects using the severity scale in Appendix, Exhibit A. Each Side Effect was ranked for each of four time periods. We then averaged each side effect for each time period and analyzed their patterns over time.
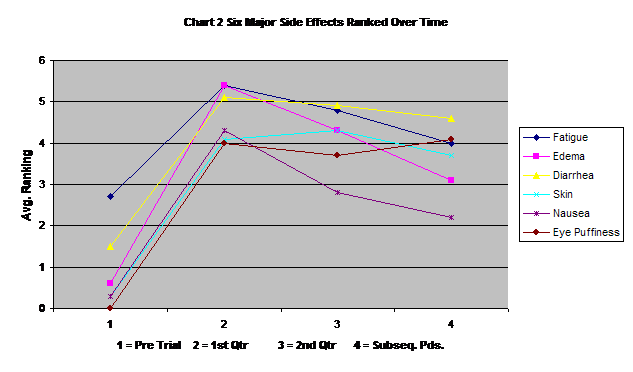
Chart two displays the changes in time of the six major reported side effects. Most had some incidence prior to the start of Gleevec and increased sharply in the first three months of Gleevec use. Following the end of the second quarter, all but eye puffiness seem to level off or decrease.
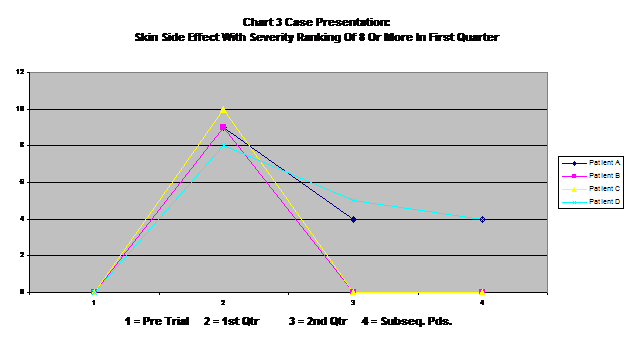
Chart three is a case study of those patients who reported very severe skin side effects in the first quarter (rating of 8 to 10). All of these showed substantial improvement over time, with two resolving completely. This seems to indicate a fairly common pattern with other severe side effects, with side effects beginning to level off and decrease after they reach a peak. Some reach this peak in the first quarter, but some seem to reach it later on.
This does not mean, however, that all is well after a period of time. Many patients have to live with significant side effects for the foreseeable future.
Side Effects Correlated With Dosage, Gender and Weight
Because of small numbers, we were unable to compare separate doses of 600 versus 800 mg per day and had to combine these into 400 versus 600 plus.
As was the case with our first Side Effects Survey, it is clear that the severity of side effects is closely correlated with dosage and with gender. As we are looking at dosage at the start of the trial and as many of the higher doses were subsequently decreased, our data most likely significantly underestimates the impact of higher dose levels.
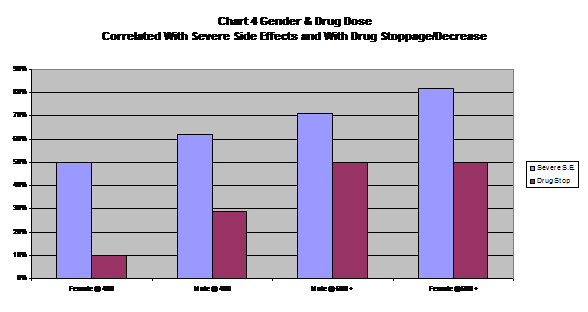
For the first time, we looked at the weight of the patient at the start of the trial to see if this was related to the severity of side effects. It was not, with both weight categories we created having identical results. (See Table 6 and Chart 4).
| Table 6 Gender, Dose and Weight (n = 61) | ||
| Gender &Starting Dosage | % With SevereSide Effects | % RequiringDrug StoppageAnd/Or Drug Decrease |
| Totals | 74% | 44% |
| Gender and Dosage | ||
| Female @ 400 mg/day |
50% |
10% |
| Male @ 400 mg/day |
62% |
29% |
| Male @ 600 plus mg/day |
71% |
50% |
| Female @ 600 plus mg/day |
82% |
50% |
| Weight At Start Of Drug | ||
| Under 150 Pounds |
74% |
46% |
| Over 150 Pounds |
74% |
48% |
Side Effect Information, Medical Management and Accuracy Of Patient Reporting
We asked each patient to rate the importance of different sources of information about side effects. As expected, the primary source was the Life Raft Group, but this is a biased result given that these patients are Life Raft Group members. Trial doctors and nurses were the most significant medical sources of information, with local oncologists playing a much less important role and local primary care physicians almost out of the picture. (See Table 7).
| Table 7 Patient Rating Of Sources Of Information About Side EffectsOn Scale Of 0 To 10, With 0 Being Totally IrrelevantAnd 10 Being Absolutely Indispensable (n = 60) | |
| Trial Doctor |
6.1 |
| Trial Nurse |
5.9 |
| Local Oncologist |
4.0 |
| Local Primary Care Physician |
2.4 |
| Life Raft Group |
8.5 |
Role Of The Trial Doctor
We asked each patient to assess the role of the trial doctor both as a source of information about, and as a manager of, their side effects. It is striking that less than half considered their trial doctor to be their primary source of information about side effects and that 25% did not consider their trial doctor to be their primary source for the medical management of their side effects. (See Table 8).
| Table 8 Patient Perception Of Role Of Trial Doctor (n = 60) | ||
| As Primary Source Of Side Effect Information * |
As Primary Provider Of Side Effect Medical Management | |
| Yes |
44% |
75% |
| No |
54% |
20% |
| N.A. |
2% |
5% |
|
100% |
100% |
|
*Where the trial doctor received the same rating as another category, the trial doctor was considered to be the primary source.
Quality Of The Medical Management Of Side Effects
We asked each patient to rate the quality of the medical management of their side effects. On a scale of 0 to 10, with 10 being the highest, the average for all doctors was 6.3. For facilities with 3 or more patients represented, three received a rating of 7 or above. Facility F received a rating of only 3.2.
The Life Raft Group held an open debate as to whether to publish the names and ratings of all the doctors or facilities, including those who rated poorly. There was not clear consensus and some were concerned about repercussions to themselves should we do so. As a result, a decision was made not to identify any facilities. (See Table 9).
| Table 9 Rating Of Quality Of The Medical Management Of Side Effects (n = 56) | |
| Facilities With 3 Or More Patients Represented | On A Scale Of 0 to 10, With 0 Being Nonexistent and 10 Being Extraordinary, How Would You Rate The Care You Have Received From Your Clinical Trial Doctor & Staff In Managing Your Side Effects? |
| 7 Or Above | Facilities A, B and C |
| 6.0 | Facility D |
| 5.6 | Facility E |
| 3.2 | |
Facility FTotal, All Trial Doctors/Facilities Regardless Of The Number Of Patients Represented
6.3
Total, All Facilities
Correlation With Patient Concerns and With Patient Rating Of The Quality Of Medical Management Of Side Effects
We asked each patient whether they were concerned about being taken off the trial because of side effects and 48% reported that they were. We then correlated this level of concern with whether the patient would consider minimizing the reporting of their side effects to avoid being taken off of Gleevec. As might be expected, there was a clear correlation, with 72% of those who were concerned about being taken off the trial reporting that they might (yes or
maybe in response to our question) consider minimizing their side effects to remain on Gleevec.
We then correlated the relationship between their medical management rating and whether the patient would consider minimizing the reporting of their side effects. The results were quite striking. The lower the medical management rating, the higher the likelihood that the patient would consider minimizing the reporting of their side effects. Only 40% of those respondents rating their medical management of side effects as 7 or above would consider minimizing their side effects as contrasted to 78% of those rating their medical management as 3 or below. (See Table 10 and Chart 5).
The importance of quality medical care in helping to ensure reporting accuracy is quite significant and has long range implications for those conducting clinical trials.
| Table 10 Accuracy Of Reporting: Correlation With Patient Concerns and With Patient Rating Of The Quality Of Medical Management Of Side Effects | |||||
| Would You Consider Minimizing The Reporting Of Your Side Effects To Avoid Being Taken Off Of Gleevec? | |||||
| Yes | Maybe | Sub Total | No | N.A. | |
| Total, All Respondents |
18% |
32% |
50% |
46% |
4% |
| 48% Are Concerned About Being Taken Off Trial * |
38% |
34% |
72% |
28% |
0 |
| Side Effect Medical Mgt Rating: | |||||
| 16% Rate As 3 or Below |
22% |
56% |
78% |
22% |
– |
| 36% Rate Between 4 & 6 |
30% |
20% |
50% |
45% |
5% |
| 48% Rate As 7 Or Above |
7% |
33% |
40% |
56% |
3% |
* In response to our question, “Have you ever been concerned about being taken off of Gleevec because of side effects,” 36% responded
yes and 12% responded
maybe (n = 60).
Methodology
The survey form used was developed over a period of time, critiqued by the Life Raft Group Science Team and a number of clinical researchers in the United States and Europe, and pre-tested by 10 Life Raft Group Patient Volunteers.
Side Effect Rating and Description
Respondents were asked to describe and rank each of their side effects using the severity scale in Exhibit A. Each side effect was ranked for each of four time periods. We then averaged each side effect for each time period and analyzed their patterns over time. Side effects were rated as severe if they received an average rating of 7 or more in any given time period or if they were cited as a reason for stopping the drug or for lowering its dosage level (See Exhibit B).
Exhibit A
| Side EffectsDescribe and rank each side effect. In addition to issues that make you feel bad (previously reported issues have included skin rash & burning, fatigue, insomnia, edema-including eye puffiness, diarrhea, reflux, nausea and cramping), also list any significant blood test abnormalities such as white blood cell, hemoglobin, neutrophils, bilirubin and platelets, and any emergency issues such as GI bleeding. | |||||
| List Each Side Effect Below.If more than five, use the Remarks Section on page 8. | Using The Severity Scale In The Right Column As A Reference, Rate Each Side Effect On A Severity Scale Of 0 Through 10 In The Time Periods Indicated Below |
Severity Scale For Rating Side Effects0 None12 Mild345 Moderate67
8 Severe 9 10 Unbearable |
|||
| Side Effect | Prior To Starting Gleevec | First 3 Months On Trial | Second 3 Months On Trial | Subseq. Periods On Trial | |
| 1. | |||||
| 2. | |||||
| 3. | |||||
| 4. | |||||
| 5. | |||||
Exhibit B
| Gleevec Dosage | ||||
| Start Date | Change Date | Change Date | Change Date | Change Date |
| Start Dosage | New Dosage | New Dosage | New Dosage | New Dosage |
| In The Appropriate Date Column, Indicate Reason For Dosage Change Or For Being Taken Off The Drug.Reported reasons have included GI Bleeding, Hepatoxicity (liver), Neutropenia (neutrophils), Infection, Edema (fluid retention) and Hematological (blood) toxicities such as WBC (White Blood Cells), HGB (Hemoglobin) and PLT (platelets).If you need more room, use the Remarks Section on page 5. | Reason | Reason | Reason | Reason |
Finally, each respondent was also asked to describe the functional effect of their side effects.
Exhibit C
Functional impact of side effects
Describe if side effects have had any impact upon what you do (Sleep, Appetite, Mood, Exercise and Other Physical Activities, Relationships (Social and Sexual), Concentration, AbilityTo Work, etc.)?
Exhibit C Comparison Of Side Effects Assessment
By Life Raft Group As Compared To That Of Novartis
Using the latest side effect data published by Novartis in a memorandum to pharmacists dated August, 2001, we compared the frequency of reported side effects to Gleevec by the Life Raft Group to those reported by Novartis. The Novartis data refers only to patients using Gleevec for Chronic Myelogenous Leukemia. The Life Raft Group data refers only to patients using Gleevec for GIST.
For most side effects, the data is fairly similar, with the Life Raft Group reporting a higher amount of fatigue, diarrhea and skin problems, and the Novartis Group reporting a higher amount of edema, nausea and cramping. Reported reflux was about the same. Only one side effect, namely eye puffiness, was reported by the Life Raft Group and not by Novartis, although that might be explained by a different method of categorization. (See Chart 6).
Where there are significant differences is in the reporting of the severity of side effects, with the Life Raft Group tending to define a greater number of side effects as severe.
There are two major reasons for these differences.
The first is that Novartis, like all clinical researchers, uses the toxicity standards established by the NCI (National Cancer Institute), whereas the Life Raft Group uses its own patient severity rating scale.
The second is that Novartis, like all clinical researchers, derives its data from the reports of on line clinicians, whereas the Life Raft Group derives its data directly from the patient. This raises the issues of whether the on line clinician correctly hears, interprets and records what the patient says and whether the patient chooses to accurately report their side effects to this person. It also raises the issue of whether the Life Raft Group volunteer who records this information correctly hears, interprets and records what the patient says and whether the patient chooses to accurately report their side effects to this person.
Although we have no Novartis data on GIST-Gleevec severity, we do have the reports of the clinical researchers at the May, 2001 ASCO (American Society of Clinical Oncologists) Conference. As is the case with all clinical trials, the GIST-Gleevec trial researchers used the NCI toxicity standards, with grades 3 and 4 events being reported by the U.S. trials and grades 2, 3, and 4 being reported by the European trials. Let’s compare the U.S. report, using grades 3 and 4 to define toxicity, to the Life Raft Group methodology. We will use as an example the fact that a clinical trial patient has diarrhea.
The U.S. ASCO Report notes that, of 145 study participants, there were 0 cases of diarrhea as a grade 3 or 4 toxicity event. The Life Raft Group notes that, of 61 study participants, there were 13 cases of diarrhea reported to be severe.
Let’s take a look at how this difference in reporting could occur.
Assume that a patient had three incidents of diarrhea per week prior to beginning Gleevec.
Assume that patient now has three incidents per day since beginning Gleevec.
Using the current NCI toxicity scale that situation would rate a one, on a scale of 0 to 5, with 0 being none and 5 being death.
Using the Life Raft Group Severity Scale, the patient might conclude that three episodes a day of diarrhea for the indefinite future, might rate a 7, on our scale of 0 to 10, thus qualifying for a ranking of severe. One might ask the NCI analyst who constructed the scale: How would you rate the severity of having to cope with an average of 3 episodes of diarrhea per day for the rest of your life?
Given that about half of those among the Life Raft Group who report severe side effects, report three or more, this poses an interesting dilemma. How do you combine the several different side effects into one quality of life whole? To some extent we have begun trying to do this by looking at functional effects, in addition to rating side effects separately. (See Table 4).
At some later date, we may return to this subject in greater detail. Our purpose today was to point out the different perspectives of a patient group and a clinical trial group in looking at the severity of side effects, and to suggest that both may help understand what a patient is actually going through as they try to live their life.