updated 7/21/2023
 Please note – Whether you have already had biomarker (aka mutational testing), or you have heard about it from others, it can be very confusing wading through the significance of the diagnostic tools for GIST. For help navigating through the terminology and treatment questions throughout your GIST journey you can call our Patient Registry Department at 973-837-9092 (or email: patientregistrydepartment@liferaftgroup.org.
Please note – Whether you have already had biomarker (aka mutational testing), or you have heard about it from others, it can be very confusing wading through the significance of the diagnostic tools for GIST. For help navigating through the terminology and treatment questions throughout your GIST journey you can call our Patient Registry Department at 973-837-9092 (or email: patientregistrydepartment@liferaftgroup.org.
The Importance of Biomarker (Mutational) Testing
Why is biomarker(mutational) testing so important? Knowing the driving force behind each individual’s tumors is not just important, it’s critical. Despite this, GIST biomarker/ mutational testing rates are poor, with only about 6% of patients having received a test in the US in 2010, and although it’s possible that percentage has increased a little bit since then, we still have a long way to go. Even in the Life Raft Group Patient Registry, a group of extremely proactive patients, only 53% of patients know their mutation.
What do I need to consider as a GIST patient? Having biomarker/mutational testing done is the most important step your oncologist can take to determine the best possible targeted treatment. When the specific mutation driving your cancer is known, the doctor can personalize your treatment, and assure that you are receiving the most effective standard of care.
We talk about GIST patient concerns in this video:
GIST Patients talk about testing here:
All GIST patients being considered for drug therapy should have biomarker (mutational testing).
Why is there such confusion over the terms ‘biomarker testing’, ‘mutational testing’, or ‘genomic sequencing’?
There are several terms used for the tests to determine what mutation or biomarker is associated with a particular cancer. The differences in terminology refer to a wide array of cancer screening panels and their focus and range of testing. GIST is not a one-size-fits-all type of cancer. There are several types of mutations that cause GIST. Each patient is an individual, and there are many factors that go into deciding what tests you should have.
We talk about terminology in cancer testing in this video:
What is a biomarker?
A biomarker is a characteristic of the body that you can measure that can be used for diagnosis, prognosis, or treatment of a disease. Cancer biomarkers can include gene mutations (changes), gene rearrangements, proteins, extra copies of genes, missing genes and other molecules. 1
Biomarker testing refers to the analysis of neoplastic tissue to look for relevant mutations, genetic alterations, proteins and other things that can be analyzed and/or measured. Biomarker testing is used to identify unique DNA alterations or changes within cancer cells that determine how a tumor behaves and grows and what therapies might be used to treat it.
Identifying Mutations and Diagnosis
When an oncologist knows the specific mutation(s) or biomarkers that are driving your cancer, they can personalize your treatment and assure that you are receiving the most effective standard of care. And although testing at the beginning of one’s treatment journey is important, it may also be done again if the cancer recurs, as it may be a different mutation that is driving new cancer growth.
A driver mutation is a mutation within the cancer that is believed to have a key role in a specific cancer. These mutations occur from damage to genes in an individual cell during an individual’s life and for GIST the cause of that damage is unknown. Mutations are changes in genes, and these changes may cause cancer by making proteins function abnormally causing unregulated cell division.
Different types of mutations can cause different types of cancer. Mutational testing is a type of biomarker testing that is based on DNA sequencing of genes. The information gathered can help to guide diagnosis, determine prognosis and to predict the course of your disease.
Biomarker Testing is Important for GIST Patients
Mutational Testing and Treatment in GIST
The first step in confirming a GIST diagnosis is immunohistochemistry which is staining to identify the expression of specific proteins by tumor cells. Positive staining for the KIT and DOG1 proteins is a diagnostic marker of GIST. After your pathologist has confirmed your GIST diagnosis, the pathologist will send the results to your oncologist. After assessing your risk/stage, they will recommend next steps for your treatment. Surgery is the gold-standard treatment for GIST. If your tumor(s) is resectable, then surgery will be performed.
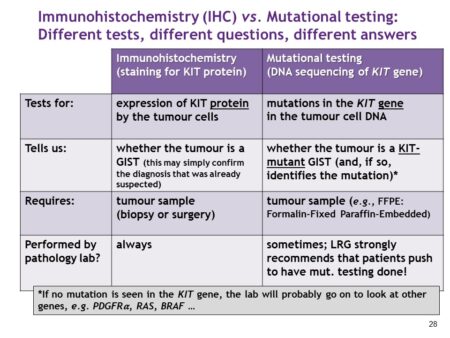
After surgery, if adjuvant therapy is recommended, biomarker testing (mutational testing) is required to identify your mutation type and to ensure that you are on the right treatment at the correct dosage. The most common type of biomarker/mutational testing is that which tests for specific genes or biomarkers.
About 15% of GIST patients will not have a mutation in the KIT or PDGFRA gene. If the results from this test show no mutation in KIT or PDGFRA (also known as wildtype or KIT/PDGFRA WT) then advanced biomarker testing should be performed to find the oncogene that is driving your GIST. There are several subtypes of GIST and newer targets are discovered all the time. There are newer forms of advanced testing like circulating tumor DNA (ctDNA) that will help to understand the complexity of your disease and which types of GIST will respond best to different treatments.
Advanced testing analyzes a wide range of genes (~ 200 – 800 genes). The most common mutations/biomarkers of this test are: SDHx, NF1, BRAF, or sometimes a gene fusion called NTRK. In associated GIST mutations, like SDH, genetic counseling for an inherited mutations is also strongly advised. The Life Raft Group can direct you to more detailed information about GISTs that are negative for KIT and PDGFRA mutations and how to advocate for the testing..
- Source: mycancer.com/resources/what-are-biomarkers
How to Order a Basic Mutational Test
Requests for KIT and PDGFRA mutation screening must originate from a pathologist or treating physician. One paraffin block of the tumor (either biopsy or surgical specimen) or about 10 unstained tissue sections of the tumor are typically needed (verify with lab). For further information on how to get a mutational test, please contact our Senior Director of Data Management & Research Denisse Evans at 973-837-9092 or devans@liferaftgroup.org
Additional Reading:
Article – Mutational Testing and Genetics
Additional Resources:
Complete Resource List
Not all these services may be available internationally. Please call the LRG if you need guidance on getting tested.
Non-Academic Laboratories
Foundation One
- FoundationOne CDx (324 DNA genes interrogated from a tissue sample)
- FoundationOne Liquid CDx (324 DNA genes interrogated from a simple blood draw)
- FoundationOne Heme (406 DNA and 265 RNA genes interrogated from a variety of sample options)
Neogenomics
- NeoTYPE® GIST and Soft Tissue Tumor Profile
The NeoTYPE GIST/Soft Tissue Tumor Profile analyzes 43 biomarkers through a combination of next-generation sequencing (NGS), FISH, and IHC. - Full Comprehensive Cancer Panel (Germline)
Tempus
Provides a broad range of DNA and RNA sequencing services, generating high-quality somatic and germline molecular data
TempusXT (Targeted Panel of 648 genes; enriched for clinically relevant genes and genes of emerging clinical relevance
TempusXE (Whole Exome of ~20,000 genes; providing the broadest genomic coverage for analysis)
Biomarker Analysis by Tumor Type
All biomarkers test includes: Next Generation Sequencing (NGS), Whole Transcriptome Sequencing (WTS), and Immunohistochemistry (IHC)
Invitae
- Invitae Familial Gastrointestinal Stromal Tumor Syndrome Panel (Germline)
Analyzes genes associated with familial gastrointestinal stromal tumor syndrome (GIST), which is a rare hereditary gastrointestinal cancer predisposition syndrome. - Invitae Multi-Cancer Panel
The Invitae Multi-Cancer Panel analyzes 84 genes associated with hereditary cancers across major organ systems
ARUP Laboratories
Gastrointestinal Stromal Tumor Mutation
- Detect activating mutations in KIT and PDGFRA. Predict response to tyrosine kinase inhibitor (TKI) therapy (2 genes tested: KIT & PDGFRA)
- Hereditary Gastrointestinal Cancer Panel, Sequencing and Deletion/Duplication
Recommended test to confirm a diagnosis of hereditary gastrointestinal (GI) cancer in individuals with a personal or family history of GI cancer and/or polyposis. When a relative has a previously identified pathogenic variant (22 Genes tested)
Labcorp
Gastrointestinal Stromal Tumors (GISTs) c-KIT Mutation Analysis with Reflex to PDGFRA Mutation Analysis
Academic Laboratories
• Oregon Health Science University **
• National Institute of Health
• Memorial Sloan Kettering Cancer Center
• University of California San Diego
• Dana-Farber Cancer Institute
• Sylvester Comprehensive Cancer Center in Miami
**The Life Raft Group could assist you in the testing process
