Masitinib was among clinical trials presented at ASCO this year. Below are a list of the others.
Advanced First-Line Patients
1. Masitinib Phase 2 & 3
Two posters were presented by principal investigators in the Phase 2 and 3 trials of masitinib for the treatment of newly diagnosed GIST patients.
Axel Le Cesne, MD at Institut Gustave Roussy, Villejuif, France, presented a five-year update of the Phase 2 masitinib trial. Patients with newly diagnosed inoperable GIST received 7.5 mg/kg/day. No prior imatinib therapy was allowed. Objectives included response rates, progression-free survival (PFS) and overall survival (OS). Thirty patients were admitted to the study. Mutation analysis was reported for 19 of the 30 patients. Median PFS was 41 months and median OS was 65 months. Five patients had prolonged response. Of these, two had a partial response and three a complete response. Exon 11 patients tended to have better response. There was no imatinib comparison arm, but these results compared favorably with results reported for the Phase 3 imatinib trial in the United States.
One caution is that there were no exon 9 patients reported in the mutation anal-ysis so the number on trial is unknown. Adverse events were reported as mild with the most common being asthenia (87 percent), diarrhea (57 percent), eye-lid edema (57 percent), nausea (47 per-cent), muscle spasm (43 percent) and rash (37 percent). The authors state that adverse events occur mainly during the first year and that there is good long term tolerance after that. They also con-clude that the results support the Phase 3 comparison with imatinib for first-line treatment of GIST.
In another poster, Antione Adenis, MD from Centre Oscar Lambret, Lille, France, reported the outline of the Phase 3 trial comparing masitinib with imatinib for newly diagnosed GIST patients. Dr. Adenis presented data showing that masitinib is the most selective kinase inhibitor under development or already approved. If kinase inhibitors were like rifles shooting at targets, masitinib has a very tight pattern centered on c-KIT like kinases whereas sunitinib looks more like a shotgun pattern with hits all over the kinase family target. This more focused approach is expected to result in fewer side-effects. The trial randomizes patients in a 1:1 ratio to 7.5 mg/kg/day masitinib or imatinib 400 mg or 600 mg. Patients remain on assigned drug until either progressive disease by RECIST or discontinuation due to toxicity.
Patients who have exon 9 mutations and who are considering this trial should be aware that the most effective dose in preventing primary resistance in Exon 9 has been 800 mg. Cross-over from imatinib to masitinib is not permitted. It is assumed that patients who fail masitinib will have access to imatinib outside the trial. There are 222 patients planned. As of May 23, 2012, 117 patients had been accrued worldwide in Western Europe, Hungary, Lebanon, Thailand and the United States.
From the poster, we learned one thing about this trial that sets it apart from other GIST trials to date. The primary objective measurement is progression-free survival. However, the trial design is not superior PFS. The design is a “non-inferiority hypothesis (85% power using a 95% two-sided confidence inter-val of the hazard ratios.)”
That is a bit complicated. First, this does not mean that masitinib is necessarily inferior to imatinib first-line in advanced GIST but that the trial can continue even though the intermediate results do not show superior PFS for masitinib. (The poster reported that the Data Review Committee did review the trial results as of May and approved continuing the trial.) In the end, masitinib can succeed if through the final trial re-sults it can be assured that 95 percent of the time it delivers 85 percent or better PFS compared to imatinib.
The second important measure in this setting will be side-effects. Masitinib can succeed as inferior at avoiding progression but at the same time superior in toxicity. Secondary endpoints include “Quality of life assessment” analysis, which will be watched closely in future trial reports. It is important to note that nilotinib did not succeed in a Phase 3 superiority trial design against imatinib. At the same time it was widely reported that patients experienced fewer side-effects on nilotinib. Now, one can only speculate if nilotinib would have succeeded in a non-inferior design. Non-inferiority trials are uncommon but can have important non-PFS endpoints in different settings. Lower costs to deliver, easier to distribute and administer (think oral versus IV) and reduced follow-up care are important everywhere but especially in the low-income countries where they can mean the difference between treatable and non-treatable on a large scale. This can be a learning experience and hopefully we will learn more as the results of this trial are available.
There were a number of posters reporting retrospective analysis of subjects that can impact GIST management. The subjects were grouped around surgery and imatinib therapy.
2. Surgery
The role of surgical cytoreduction before imatinib therapy in patients with advanced GIST. Hojung an, MD of Division of Medical Oncology, Asan Medical Center, Seoul, South Korea. In this poster reporting results of an analysis in Korean patients treated from 2001 to 2010 the authors conclude that tumor burden on diagnosis is an inde-pendent predictor of survival after imatinib therapy. Although patients who had cytoreduction tended to have less progression the authors also conclude that surgery before starting imatinib in newly diagnosed advanced GIST does not significantly benefit prognosis. Notably, the group of patients having surgery was small (35) compared to those who did not (235).
Outcomes of multivisceral resection of gastric gastrointestinal stromal tumors. Sabha Ganai, MD University of Chicago Medical Center, Chicago, Illinois. Here the authors report that pa-tients with larger tumors tend to have more than one organ (multivisceral) involved in their surgery. The history of patients at the University of Chicago indicates that these patients have significantly earlier progression and lower proportion of survival at three years post-surgery. They note that the 73 patients analyzed from 2001 to 2007 in this study represent a wide range of presentations (clinical heterogeneity) and that GIST management practice patterns changed over this time.
Neoadjuvant treatment of locally advanced GIST: Results of APOLLON, a prospective, open label Phase II study in KIT- or PDG-FRA-positive tumors. Peter Hohenberger, MD Mannheim University Medical Center, Mannheim, Germany.
This discussion poster presented the results of a Phase 2 trial in Austria and Germany sponsored by the Technische Universität München. The trial started in 2005. Forty-one patients with locally advanced primary GIST (clinically non-metastatic) were given imatinib for six months followed immediately by surgery. Many of these patients were judged inoperable or requiring multivisceral resection upon at the outset of treatment. Multivisceral resection is multi-organ surgery i.e. sur-gery impacting stomach and spleen or other organ combinations. The primary endpoints were PFS at one and five years. Secondary endpoints included the rate of organ-preserving surgery. The study concluded that “Neoadjuvant therapy with imatinib 400mg/day for six months is safe and downsizes tumors significantly. Firstline multivisceral resection of primary GIST should no longer be the standard of care.” In the United States the National Comprehensive Cancer Network (NCCN) guidelines currently caution that complex multivisceral resection should be avoided in primary GIST and suggest perioperative imatinib therapy treatment with a multi-disciplinary team approach.
The chart with this article (Figure 1) was prepared from data in the poster. It shows the greatest improvement for initially inoperable patients 93 percent of whom could eventually have surgery. Significant improvements in morbidity were also seen for patients who initially required either multi-visceral resection or full organ removal.
Dr. Hohenberger compares his study to a similar RTOG study in the US. In both studies of mostly high risk patients five-year PFS was over 56 percent, while OS was over 77 percent. These results are described as promising since both studies took place in the era before adjuvant therapy was fully adopted. These studies are small. Ideally they will be repeated under the latest guidelines for GIST manage-ment. It would be helpful to see outcomes by mutation type and with longer adjuvant therapy.
3. Imatinib therapy
Pharmacokinetics of escalated dose of imatinib in patients with advanced gastrointestinal stromal tumors. Changhoon Yoo Asan Medical Center, University of Ulsan College of Medicine, Seoul, South Korea.
This poster is a retrospective study of 66 advanced Korean patients who from 2008 to 2011 experienced resistance on 400 mg imatinib and increased dosage to 800 mg. Imatinib minimum concentration levels (Cmin) were taken at 400mg and after escalating the dose to 800 mg. The absolute and percent changes in blood levels as well as tumor response were measured. The authors conclude that “Imatinib Cmin at 800 mg and the percent change of Imatinib Cmin were not associated with response and survival outcomes.”
This report differs significantly from other studies of Imatinib Pharmacokinetics (PK) (often referred to as imatinib blood levels or trough levels). It studies PK in patients who have already experienced progression. Most studies to date have looked at PK in recently diagnosed advanced patients who have not progressed and for whom the question is “would management of PK help avoid progression.” So the results of this study are not necessarily applicable to the pre-resistance setting.
This study included a relatively high ratio of KIT exon 9 GIST patients (28 percent) who do not respond as well to 400 mg imatinib. Exon 9 patients typ-ically experience a better initial response to higher dose (800 mg) than to lower dose (400 mg) imatinib. However in this exon 9 enriched cohort, where one would expect to see some survival improvement attributable to overcoming primary resistance there was none reported. One possible reason may be the distribution of Cmin values reported for these patients at the lower 400 mg dose. The distribution indicates many patients were already at 1,100 ng/ml Cmin or higher before starting imatinib 800 mg. If this is true then a lower rate of response might be expected in these patients. The lack of response noted in the results might therefore be questioned on these bases.
In additional findings, the authors highlight the correlation between body surface area and Cmin levels at 800 mg. An association was also found between severe toxicities and percent change of imatinib Cmin following dose escalation. In conclusion they suggest that monitoring of imatinib Cmin might help to predict or manage toxicity induced by escalated dose of imatinib.
Observational/Retrospective reports with potential impact on GIST management.
Diagnosis and initial evaluation of patients with gastrointestinal stromal tumor (GIST): An observational study of 1,226 patients. Dr. Jonathan Trent University of Miami Sylvester Comprehensive Cancer Center, Miami, FL.
There were 1,226 patients accrued between January 2005 and December 2010. During this period GIST management practices changed significantly. Twelve-month adjuvant IM treatment began as an accepted practice in June 2007 and was FDA-approved in December 2008. A third or more patients en-tered the ReGISTry study before adjuvant treatment was accepted. Thirty-six month adjuvant treatment was accepted standard after June 2011. The ReGISTry data now represents more a window on past treatment practices. As an example, in this update only 8 percent of patients accrued are reported to have had muta-tion analysis. More recent patient series show a higher percent of patients in the U.S. are receiving mutation analysis after 2009.
The data highlighted in this poster (Figure 2) concerned the mix of specialties diagnosing and then managing GIST. Surgeons are primary at diagnosis, while medical oncologists are primary for management after diagnosis. The data points out the importance of a multi-disciplinary team approach to managing GIST.
Characteristics of gastrointestinal stromal tumor (GIST) patients receiving short-term versus long-term imatinib (IM) adjuvant therapy: A chart review analysis. Annie Guerin Analysis Group, Inc., Boston, MA
The authors of this study included analysts at Analysis Group a healthcare consulting service, employees of Novartis Pharmaceuticals and Anthony Conley, MD of Moffitt Cancer Center in Tampa, Florida. A second consulting firm (All Global) was used to contact oncologists in the United States who were recruited to use their physician administered on-line patient chart reviews. Three hundred twenty physicians contributed data. Of these, 98 percent were hematologists/oncologists or medical oncologists. Only four self-described as sarcoma specialists, while 22 percent were in institutions and 77 percent were in private practice. Most were in small to intermediate-sized practices (two to nine physicians).
Data was collected on 819 GIST patients who underwent surgery as primary treatment after December 19, 2008, the date one-year adjuvant imatinib treatment was approved by the FDA. The patient data was divided into two groups: a short term group—those treated continuously with imatinib for six to 12 months following surgery (n=411), and a long term group—those treated with imatinib continuously for at least 24 months (n=408).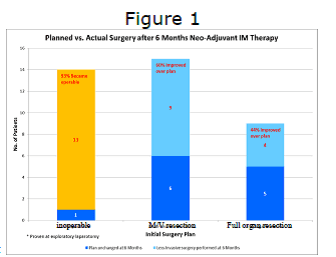
Data was collected on patient, physician and clinical outcome characteristics.
Findings included:
—Patients in the long-term group tended to be higher risk.
—Patients in the short-term group had more co-morbidity (cardiovascular and ischemic heart disease)
—Despite the higher risk characteristics of the long-term group the study found that:
—Imatinib use over an extended period of time was associated with lower recurrence rates and lower mortality rates
—After controlling for confounding factors the risk of recurrence was 4.67 times and the risk of mortality was 3.74 times higher in the short term versus the long term.




