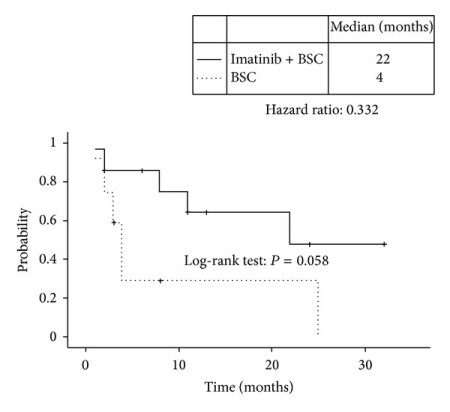
A recent nonrandomized study has shown that imatinib rechallenge impacted the survival rate for patients with advanced or metastatic gastrointestinal stromal tumor (GIST).
The study was comprised of Twenty-six patients who had previously been exposed to both imatinib and sunitinib. Patients were able to choose between a regiment of Best Supportive Care (BSC) with or without imatinib, based on physician recommendation. Fourteen patients were treated with imatinib plus BSC and 12 received BSC alone. The overall survival was greatly improved for the imatinib group. Three patients (21%) had a clinical response in the imatinib group, and one had a clinical response in the BSC alone group.
The following chart shows the median overall survival in the imatinib plus BSC and BSC groups, respectively, was 22 months and 4.0 months:
Overall survival curves in the imatinib plus BSC group (solid line) and the BSC alone group (dotted line). Median overall survival times were 22 months and 4 months, respectively. The hazard ratio for imatinib plus BSC was 0.332 (P = 0.058, log-rank test).
While the comparison between the two groups was not technically found to be statistically significant, it does highlight the importance of continuing imatinib treatment for GIST even after progression. A larger study would require a no treatment (possibly placebo) arm. The place such a study would be of benefit is in countries that currently don’t allow imatinib upon progression, as those patients wouldn’t have access to the drug in any case.
Visit this NCBI article for full details on the imatinib rechallenge study.
References:
Akira Sawaki, Tatsuo Kanda, Yoshito Komatsu, and Toshirou Nishida, “Impact of Rechallenge with Imatinib in Patients with Advanced Gastrointestinal Stromal Tumor after Failure of Imatinib and Sunitinib,” Gastroenterology Research and Practice, vol. 2014, Article ID 342986, 6 pages, 2014. doi:10.1155/2014/342986