 Every Wednesday in 2024 will be dedicated to learning about the clinical trial process and to highlighting the groundbreaking clinical trials focused on gastrointestinal stromal tumors (GIST). We understand the importance of staying informed about the latest advancements in research and treatment options, and we want to empower you with the knowledge to make informed decisions about your healthcare journey.
Every Wednesday in 2024 will be dedicated to learning about the clinical trial process and to highlighting the groundbreaking clinical trials focused on gastrointestinal stromal tumors (GIST). We understand the importance of staying informed about the latest advancements in research and treatment options, and we want to empower you with the knowledge to make informed decisions about your healthcare journey.
Clinical trials are an essential part of medical research, aimed at introducing new procedures and treatments to the public. They help understand how medicines impact the body in terms of safety, effectiveness, and which groups would benefit most from them, considering the drug’s mechanism of action and risk analysis. Through these trials, new ways to prevent, diagnose, and treat cancer can be discovered. Clinical trials can also be supported by additional data sources such as animal studies, registries, and natural history studies, offering a comprehensive view of how a medicine functions and its response in treating specific types of cancer. Ultimately, the goal of clinical trials and drug development is to determine the safety and effectiveness of new drugs or treatments.
Clinical trials are critical for the development of new treatments – every new medicine, at some point, has to undergo clinical trial. All of standard treatments for GIST, and the ways in which they are administered were once studied in clinical trials.
Clinical trials are the reason we have Gleevec (imatinib) as a breakthrough GIST treatment.
Gleevec, also known as imatinib, is a good example of how clinical trials can successfully provide new and effective treatment options for GIST. Now the first-line treatment for GIST, Gleevec is an inhibitor of a protein family called tyrosine kinases and was initially discovered in clinical trials intended to treat chronic myeloid leukemia. Imatinib is considered a promiscuous inhibitor meaning that it doesn’t just inhibit its target but also inhibits targets that have a similar structure. The abnormal signaling in GIST results from mutations in a specific protein a family of proteins, called tyrosine kinases, that is similar to the mutant protein in chronic myeloid leukemia. This over-active protein leads the cancer to grow. Therefore, researchers thought that Gleevec could effectively treat GIST, much like it did for chronic myeloid leukemia.
The first person to receive Gleevec for GIST was a patient in 2000, who was not responding to the available treatments at the time, like surgery and chemotherapy. Within one month of beginning Gleevec treatment, this first patient demonstrated rapid response, including a reduction in tumor size, and had very few side-effects.
The success of Gleevec treatment in this patient prompted Phase I and Phase II clinical trials in the US and Europe to test the safety and effectiveness of Gleevec in treating GIST. This was quickly followed by a Phase III clinical trial to compare the effectiveness of two different doses.
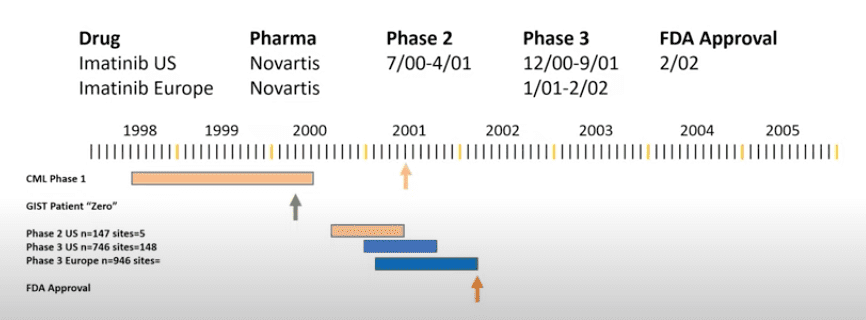
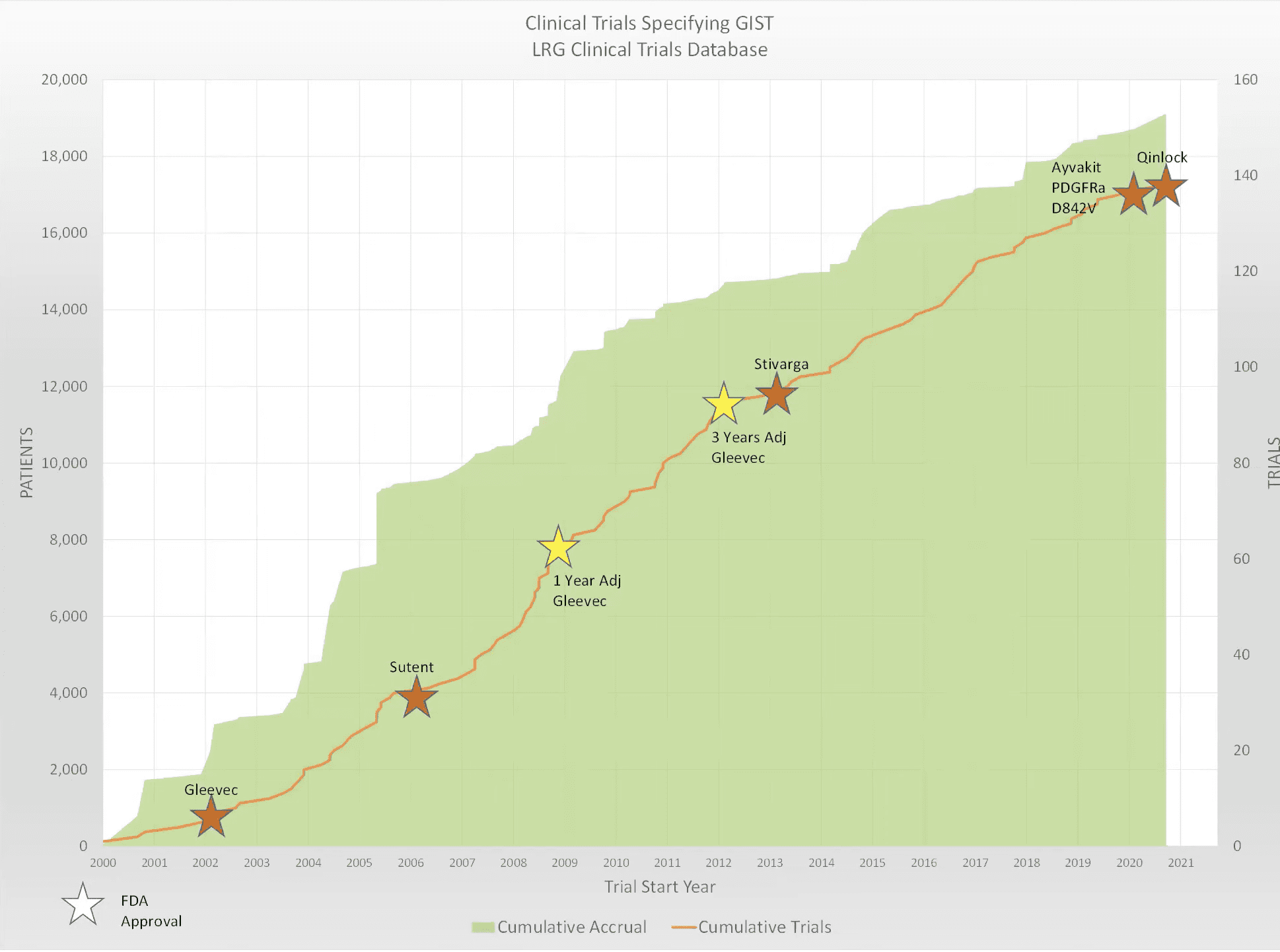
In 2002, only 2 years after the first GIST patient received it, the FDA approved Gleevec for GIST – specifically for patients with c-Kit mutations who had unresectable or metastatic malignant GIST. Before Gleevec was approved, there were not many effective treatments for GIST, especially for unresectable or metastatic GIST. After additional clinical trials, Gleevec received FDA approval for treatment of GIST: one year of adjuvant Gleevec treatment was approved in 2008 and three years of adjuvant treatment was approved in 2012. The success of these clinical trials for Gleevec completely changed the treatment options and outcomes for patients with GIST giving hope to a once-untreatable cancer. Additionally, Gleevec is also administered in neoadjuvant settings to reduce the tumor size to optimize surgical outcomes.
Clinical trials for Gleevec are still on-going. Clinical trials are still being used to determine the benefits of increasing the duration of adjuvant Gleevec treatment for GIST, and whether it can be used in combination with other drugs to further improve patient outcomes.
*Adjuvant: Adjuvant treatment refers to therapy given after the primary treatment, such as surgery, in order to lower the risk of cancer recurrence.
Additional GIST treatments are also thanks to clinical trials.
Not only did clinical trials lead to Gleevec as the first treatment (first-line) for GIST, but they have also helped to find other drugs that can be used if Gleevec doesn’t work or if its effectiveness declines. Drugs like Sutent (or sunitinib), Stivarga (or regorafenib), Ayvakit (avapritinib), and Qinlock (or ripretinib) are all FDA approved drugs for GIST because of successful clinical trials.
Sutent (or sunitinib) and Stivarga (or regorafenib) are second-line and third-line therapies for patients who are intolerant or resistant to Gleevec. Sutent was approved by the FDA in 2006 and Stivarga was approved in 2013. Qinlock (or ripretinib), is a fourth-line treatment, approved by the FDA in 2020. Clinical trial research has helped us find these alternative treatments and their treatment sequence.
Clinical trials are also helping us to understand why some drugs work better for certain mutation types of GIST. Ayvakit (or avapritinib), for example, was found to be the best treatment for patients with PDGFRA exon 18 mutations and was FDA approved to treat unresectable and metastatic GIST with PDGFRA exon 18 mutations in 2020. Through clinical trials, it was also determined that Sutent might have an enhanced treatment effect for treating KIT exon 11 and 13 mutations, while Qinlock might have an enhanced effect treating GIST harboring KIT exon 11 and exon 17/18 mutations. Because of these discoveries, new clinical trials are now selecting patients and their treatments based off of their specific GIST mutations. These clinical trials will hopefully give us more specific, individualized and effective treatment options for all patients with GIST.
Clinical trial research is essential for finding new and improved treatments for cancers like GIST. Participating in clinical trials not only provides new treatment options for current patients, but, importantly, it also helps researchers find better treatments for patients in the future. Without the thousands of patients who have participated in clinical trials for Gleevec, Sutent, Stivarga, Ayvakit, and Qinlock, we would not have the treatment options for GIST that we have today.
If you are interested in a clinical trial and would like support, please reach out to patientregistrydepartment@liferaftgroup.org. The LRG has many options for peer-to-peer support as well including private discussion groups focused on specific mutations and trials, as well as online & in-person patient support groups and GIST Mentors available for patients and caregivers. We encourage you to take advantage of these resources. You are not alone, and we are here to help.




