Every Wednesday in 2024 will be dedicated to learning about the clinical trial process and to highlighting the groundbreaking clinical trials focused on gastrointestinal stromal tumors (GIST). We understand the importance of staying informed about the latest advancements in research and treatment options, and we want to empower you with the knowledge to make informed decisions about your healthcare journey.
Every Wednesday in 2024 will be dedicated to learning about the clinical trial process and to highlighting the groundbreaking clinical trials focused on gastrointestinal stromal tumors (GIST). We understand the importance of staying informed about the latest advancements in research and treatment options, and we want to empower you with the knowledge to make informed decisions about your healthcare journey.
Clinical trials are the backbone of medical progress, serving as the driving force behind the development of new treatments and therapies. Understanding the details of these trials is crucial, as it empowers you to make informed decisions about your healthcare, demystifies the process, and ensures you play an active role in shaping the future of GIST treatment.
Knowing Your Mutation Informs Best Treatment
By knowing your specific mutation type, you can find the most effective GIST treatment for you. Studies have shown that if you have GIST with mutations in KIT exons 11 and 17/18, ripretinib may be a better option for second-line treatment than the standard sunitinib.
Genes a Blueprint for Proteins
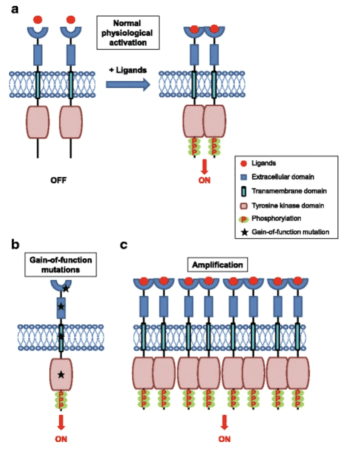
Genes are the blueprint for all proteins made in your body. Each gene is as unique as the protein it encodes for. Abnormalities in genes may be due to single or multiple mutations, leading to the production of an abnormal protein. Most patients with gastrointestinal stromal tumors (GIST) have mutations related to the KIT and/or PDGRF genes. These genes encode for proteins called tyrosine kinases. Tyrosine kinases act as the “on/off” switch for many signals in our body, including signals that tell cells to grow leading to tumor development. GIST tumors have over-active tyrosine kinases which tells the cells to grow. Most of the drugs that treat GIST are inhibitors of tyrosine kinase which work by blocking the activity of tyrosine kinases. However, different GIST drugs can be more or less effective depending on the specific GIST mutation type and the subsequent specific abnormal protein.
When tyrosine kinase is over-active, this can cause increased cell growth which can ultimately lead to the formation of tumors and cancers, like GIST. One type of mutation that leads to over-activity and production of tyrosine kinases is classified as “gain-of-function” mutations. This is when a tyrosine kinase remains active and continues to activate other proteins, even when it shouldn’t. Drugs like imatinib, sunitinib, ripretinib, and many other drugs for GIST, are tyrosine kinase inhibitors which block the activity of tyrosine kinase to prevent cancer growth. Tumors or cancers with more tyrosine kinase activity may be more sensitive to these drugs, leading to their “targeted” treatment in GIST.
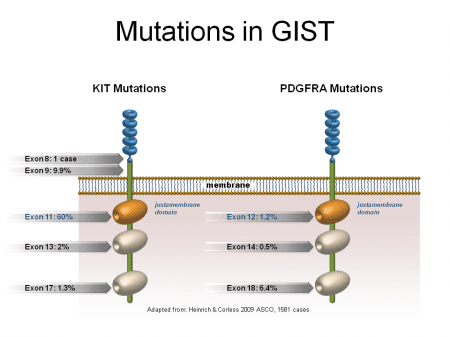
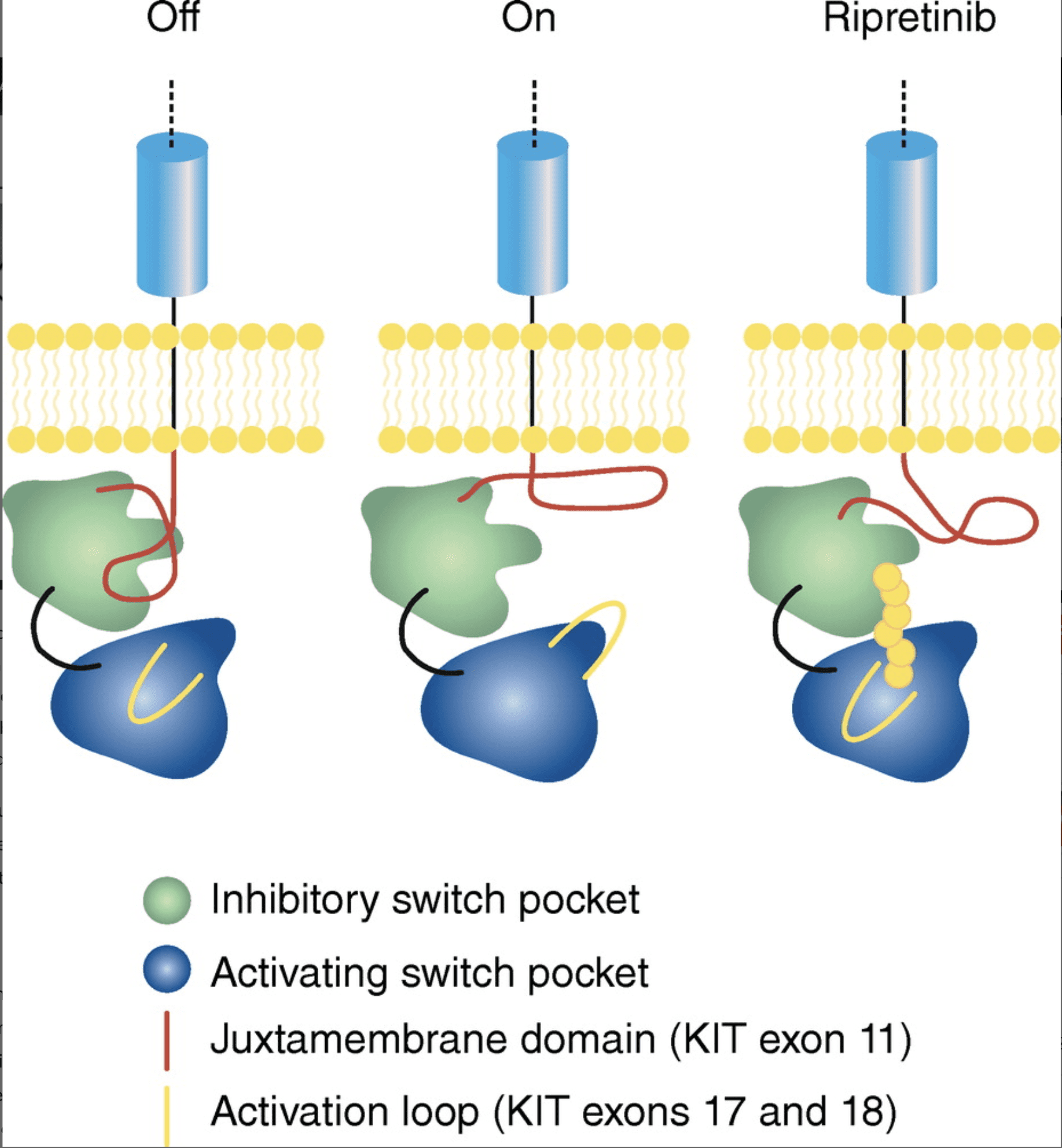
Genes are defined as segments of DNA containing information to create proteins. Different segments of a gene that instruct how a protein should be created are called exons. Exons are responsible for different parts of the protein production. Mutations to the KIT or PDGRFA genes can be caused by mutations to exonswithin the genes. Depending on which exons are mutated, different parts of the tyrosine kinase protein will malfunction. For example, mutations in KIT exon 11 (the most common primary mutation in GIST), decrease tyrosine kinase’s ability to regulate its own activity. Mutations to exon 17/18, ( less common mutation as a primary mutations, but are most common as secondary mutations*), are known for being more resistant to standard GIST drugs. Mutations to KIT exon 17 and 18 and PDGRFA exon 18 affect a part of the tyrosine kinase called the activation loop. These mutations force the tyrosine kinase to stay in its activated form, with the switch always ‘on’.
*Secondary Mutations: Secondary mutations in GISTs occur after the tumor has already developed. These mutations often occur within the same genes (KIT or PDGFRA) as the primary mutations but affect different regions of these genes. Secondary mutations can lead to resistance to targeted therapies, such as imatinib.
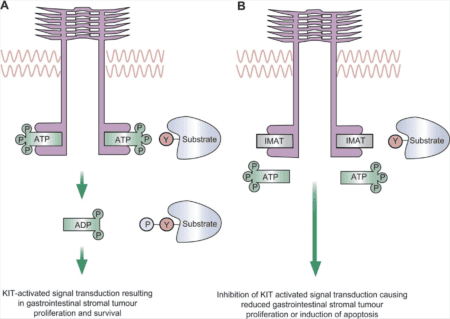
Drugs like imatinib, sunitinib, and ripretinib are all tyrosine kinase inhibitors, but they target different parts of the tyrosine kinase protein. Drugs like imatinib and sunitinib work by preventing the tyrosine kinase from binding to its energy source, called ATP, a molecule that it uses to activate other proteins. These drugs work well as first and second-line treatments for GIST with mutations in KIT exons 11.
Ripretinib, an FDA approved third-line treatment of GIST, works by binding to a region, known as the activation loop, that forces the tyrosine kinase to remain in an inactive form and decreasing the activity. Therefore, ripretinib may work better for mutations that affects the activation loop, like secondary KIT exon 17 and 18 mutations.
Ripretinib was previously found to work well as a fourth-line treatment of GIST, however, it is now being studied as a treatment for specific mutation types. Ripretinib is currently finishing a phase 3 clinical trials to investigate its use as a second-line treatment for GIST, specifically for patients who have KIT exon 11 and exon 17/18 mutations. This is an important study because most current medications, like imatinib and sunitinib, have lower levels of success at treating KIT exon 17/18 mutations. Patients with these mutations showed significantly better longer durations of progression free survival after taking ripretinib compared to sunitinib, with a progression free survival of 14.2 months for ripretinib vs. 1.5 months for sunitinib. This indicates ripretinib’s potential to be used instead of sunitinib for people with those mutations.
If you are interested in a clinical trial and would like support, please reach out to patientregistrydepartment@liferaftgroup.org. The LRG has many options for peer-to-peer support as well including private discussion groups focused on specific mutations and trials, as well as online & in-person patient support groups and GIST Mentors available for patients and caregivers. We encourage you to take advantage of these resources. You are not alone, and we are here to help.