Initial Treatment
Approximately 80% of GIST patients have only a single tumor at diagnosis (this is called a primary tumor). Surgery to remove the primary tumor is the gold standard for treatment in GIST and it offers the best chance for a cure. Surgery is the typical initial treatment after a patient is diagnosed, especially if the patient has only one tumor that can be removed fairly easily (primary surgery).
All GISTs larger than 2 cm should be resected if possible. Some GISTs have a low risk of recurrence after surgery. These are typically smaller GISTs with a low mitotic rate (see Risk of Recurrence). The management of non-rectal GISTs smaller than 2 cm with a low mitotic rate (less than 5 per 50 HPF) remains somewhat controversial. Some doctors recommend monitoring these low risk GISTs and some may recommend removal. Small rectal GISTs have a significant chance of having a high mitotic rate. Rectal GISTs smaller than 2 cm but having a mitotic rate above 5 per 50 HPF have a higher chance of recurrence and are good candidates for surgery.
Surgery for primary tumors located in some locations can be more difficult or result in more complications for the patient. In particular, tumors located in the esophagus, duodenum (sometimes requires removal of the pancreas) and rectum may result in more complications or lifestyle limitations. In these situations, a multidisciplinary review is critical. In some cases, complications or the scope of the surgery can be decreased with the use of Gleevec prior to surgery (neoadjuvant Gleevec).
- Extended anatomic resections (such as total gastrectomy) are rarely indicated.
- Since GIST rarely spreads to the lymph nodes, removal of the lymph nodes is usually not required.
- GIST tumors are often surrounded by a “pseudocapsule” and are very friable. Every effort should be made to not violate the pseudocapsule.
- Laparoscopy may be considered for some GISTs in favorable locations. Laparoscopy should be performed by surgeons with appropriate laparoscopic experience.
- Rectal GIST should be approached via a sphincter-sparing approach.
- A biopsy may be done before or after surgery.
- The goal of surgery is complete removal of the tumor with negative margins (wide margins are not necessary).
What’s the role of surgery in the management of GIST?
GIST patients have benefited from multiple FDA-approved medications for their disease. However, medication is not the sole tool for disease management – surgery is important as well. Most patients will undergo surgery at some point during their treatment. Most commonly it is used as the initial treatment after a confirmed diagnosis of GIST, especially if the patient has only one tumor that can be removed fairly easily (primary surgery). When there’s a single tumor but surgery appears to be more difficult, the patient may be given imatinib before surgery to try to shrink the tumor and make surgery less complicated. When this happens, the imatinib given prior to surgery is classified as neoadjuvant therapy.
Surgery and Metastatic GIST
In some cases, a patient presents with multiple tumors at different locations in the body upon diagnosis. In other cases, a primary tumor is removed, but a patient eventually develops additional tumors throughout the body. In both cases, the patient is said to have metastatic disease. While medication is undoubtedly important in treating metastatic GIST, surgery can be important as well.
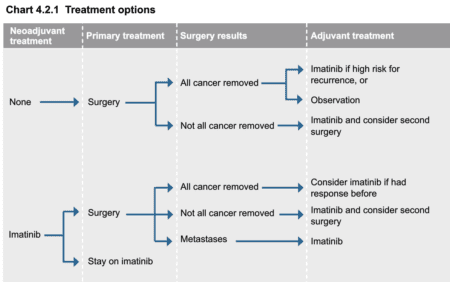
Treatment Options from the NCCN Guidelines for Patients® | Soft Tissue Sarcoma
click to view larger
Some gastrointestinal stromal tumors (GIST) may present as metastatic at the time of diagnosis. The most common site for metastases are the liver and/or peritoneum. With widespread progression (multi-organ involvement), surgery may not achieve removal of all visible tumors. In this case, the NCCN recommends treatment with imatinib as the standard of care to help prevent further recurrences. If surgery for metastatic GIST becomes viable as determined by healthcare team, resection is advisable to lessen tumor bulk/volume, although this is not potentially curative as some GIST cells may remain post-surgery.
A study by Park, et.al, (2014), demonstrated a likely benefit of surgical resection of residual lesions after disease control with imatinib. The study looked back at 134 advanced GIST patients treated with imatinib or imatinib + surgery. The imatinib group (92 patients) had a median progression-free survival (PFS) of 42.8 months versus 87.7 months in the imatinib + surgery group (p=0.001). Even more importantly, the overall survival times were longer in those receiving both imatinib and surgery (OS not reached) versus imatinib alone (88.8 months).
In the absence of big, randomized (not plausible for this type of study) clinical data, one of the most solid conclusions seems to be that surgery is of little benefit for patients with widespread progression of metastatic disease. Surgery for limited progression (one or two tumors) appears to have some benefit, and surgery for metastatic GISTs which have achieved stability with tyrosine kinase inhibitors (imatinib, sunitinib, etc.) is an individualized case that needs a multi-disciplinary collaboration with recognized GIST experts.
The decision on whether to have surgery for metastatic disease after responding to Gleevec (imatinib) is a complex decision.
It involves many factors such as:
- Can all visible disease be removed?
- How complicated is the surgery?
- How likely are complications?
Does having surgery mean I won’t have recurrence and don’t need adjuvant Gleevec?
In a few words, it’s not that simple. Recurrence can still occur and Gleevec (imatinib) is most likely needed depending on your mutation and risk of recurrence category. Read more about adjuvant Gleevec and mutational testing. Read more hear about Gleevec after surgery in our Adjuvant treatment for GIST section.
I’ve had surgery. Now what?
Surgery is an important tool in helping manage GIST, but it also can result in changes in the body that need adjustments. Particularly, as in many cases you’re dealing with the gastrointestinal tract, nutrition is one area that is often impacted significantly. Below is some information that deals specifically with nutritional issues after gastrointestinal surgery:
The removal of the stomach or other parts of the GI tract can require lifestyle changes, especially when it comes to diet. Managing and understanding how to properly nourish the body is important. Patients who undergo a gastrectomy may have to eat smaller and more frequent meals. They may have more difficulty absorbing some nutrients and may suffer from “dumping syndrome”, which is caused when food moves too rapidly into the small intestines. Almost everyone who has undergone surgery will form a type of scar tissue called adhesions. These adhesions can cause pain and in some cases bowel obstruction. Not everyone will develop these problems and if they do occur, they may not happen for many years.
Some common concerns are:
Gastrectomy and dumping syndrome
Proper nutrition after surgery
For more information on managing other side effects of GIST:
References
Blanke CD, Eisenberg BL, Heinrich MC, Gastrointestinal stromal tumors, Curr Treat Options Oncol, 2001;2:485–91.
Blanke CD, Demetri GD, von Mehren M, Heinrich MC, Eisenberg B, Fletcher JA, Corless CL, Fletcher CDM, Roberts PJ, Heinz D, Wehre E, Nikolova Z, Joensuu H, (2008). Long-term results from a randomized phase ii trial of standard- versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing kit. J Clin Oncol, 26:620–625.
DeMatteo, RP, Lewis, JJ, Leung, D, Mudan, SS, Woodruff, JM, & Brennan, MF. (2000). Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Annals of Surgery,231(1), 51.
DeMatteo, RP, Ballman, KV, Antonescu, CR, Corless, C, Kolesnikova, V, von Mehren, M, … the American College of Surgeons Oncology Group (ACOSOG) Intergroup Adjuvant GIST Study Team for the Alliance for Clinical Trials in Oncology. (2013). Long-term results of adjuvant imatinib mesylate in localized, high-risk, primary gastrointestinal stromal tumor (GIST): ACOSOG Z9000 (Alliance) intergroup phase 2 trial. Annals of Surgery, 258(3), 422–429. http://doi.org/10.1097/SLA.0b013e3182a15eb7
Heinrich MS, Owzar K, Corless CL, Hollis D, Borden, EC, Fletcher, DM, … Fletcher, CDM. (2008) Correlation of kinase genotype and clinical outcome in the North American Intergroup Phase III Trial of Imatinib for Treatment of Advanced Gastrointestinal Stromal Tumors: CALGB 1150105. Journal of Clinical Oncology;26(33):5360-5367.
Joensuu H, Eriksson M, Sundby Hall K, Hartmann JT, Pink D, Schütte J,…Reichardt P. (2012). One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor a randomized trial. JAMA;307(12):1265-1272. doi:10.1001/jama.2012.347
Park SJ, Ryu MH, Ryoo BY, Park YS, Sohn BS, Kim HJ, Kim CW, Kim KH, Yu CS, Yook JH, Kim BS, Kang YK. The role of surgical resection following imatinib treatment in patients with recurrent or metastatic gastrointestinal stromal tumors: results of propensity score analyses. [Abstract] Ann Surg Oncol. 2014 Dec;21(13):4211-7. doi: 10.1245/s10434-014-3866-4. Epub 2014 Jul 1.
Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol 2005; 29: 52–68.
Reichardt, P., Blay, J-Y., Boukovinas,I., Brodowicz, T., Broto, J. M., Casali, P. G.,…Joensuu,H. (2012). Adjuvant therapy in primary GIST: state-of-the-art. Annals of Oncology. doi: 10.1093/annonc/mds198
