Caution: Pediatric-type GIST may have a very different potential for metastases and these methods should not be used for estimating risk for pediatric-type GIST.
Many attempts have been made to classify GISTs as to their potential for malignant behavior. However, Pathologists who are GIST experts, currently think it most prudent to classify GISTs based on risk assessment, rather than try to classify them as benign or malignant. At least some GIST experts think it is unwise to use the term “benign” with GIST and that almost all GISTs should be considered as having some malignant potential.
Although many factors have been suggested to contribute to malignant potential, the three most commonly quoted factors are:
- Primary tumor size
- Primary tumor location
- Mitotic activity (also called mitotic rate or mitotic index)
Other important factors for a high risk of recurrence include:
- Rupture of the tumor either prior to or during surgery.
- Failure to obtain clear margins during surgery.
- Genotype- Deletions in KIT exon 11, especially deletions of codons 557 and 558 have been shown to increase the chance of a recurrence.
All of the risk assessment methods on this page estimate the risk of a recurrence or recurrence-free survival (GIST nomogram) for GIST patients not taking imatinib. For patients, with GIST that has a high risk of recurrence, adjuvant imatinib has been shown to reduce recurrence and increase survival when taken for three years compared to patients that only take it for one year. See Gleevec for GIST.
Pooled analysis
Using contour maps, provides a visual aid in understanding risk
This method of estimating risk of recurrence is based on a pooled analysis of 10 population-based series of over 2500 patients with operable GIST that did not receive adjuvant imatinib after surgery. Risk was stratified using the National Institute of Health (NIH) consensus criteria, the modified criteria, and the Armed Forces Institute of Pathology (AFIP) criteria.
Importantly, tumor rupture was added as a separate category. Most other systems only note tumor rupture as a higher risk; this system integrates tumor rupture into the risk assessment. This paper was published in The Lancet Oncology on December 7th, 2011 (early online edition).
Modified NIH Method
Adds tumor rupture and site to the NIH system
The modified NIH method adds several additional criteria to the original GIST Workshop (NIH) method (see table below). In addition to tumor size and mitotic rate which were factored in the original NIH criteria, it adds tumor location and tumor rupture. Most of the major methods do not include tumor rupture as a risk factor.
Note:
- High risk tumors as defined by this method have more than a 15% to 20% risk of a recurrence.
- Many patients classified as intermediate risk using other methods can be reclassified as either high or low risk when using the modified NIH method.
See commentary by Dr. Joensuu; Adjuvant Therapy for High-Risk GIST: Considerations for Optimal Management (September, 2012).
|
Modified NIH Method
|
|||
|---|---|---|---|
|
Size
|
Mitotic Count
|
Primary Tumor Site
|
|
| Very low risk |
≤2.0 cm
|
≤5/50 HPF
|
Any
|
| Low risk |
2.1-5.0 cm
|
≤5/50 HPF
|
Any
|
| Intermediate risk |
2.1-5.0 cm
|
>5/50 HPF
|
Gastric
|
|
<5.0 cm
|
6-10 HPF
|
Any
|
|
|
5.1-10 cm
|
≤5 HPF
|
Gastric
|
|
| High risk |
Any
|
Any
|
Tumor rupture
|
|
>10 cm
|
Any
|
Any
|
|
|
Any
|
>10 HPF
|
Any
|
|
|
>5.0
|
>5 HPF
|
Any
|
|
|
2.1-5.0 cm
|
>5 HPF
|
Nongastric
|
|
|
5.1-10.0 cm
|
≤5/50 HPF
|
Nongastric
|
|
| Abbreviation: HPF = high-power field. | |||
AFIP-Miettinen System
A commonly used system for estimation on individualized outcomes
In April, 2010, the Journal of the National Comprehensive Cancer Network issued the “NCCN Task Force Report: Update on the Management of Patients with Gastrointestinal Stromal Tumors” These guidelines contain both the AFIP-Miettinen method of determining risk (defined as metastasis or tumor-related death) and the GIST nomogram system. (See the table below).
These guidelines are based on data developed at the Armed Forces Institute of Pathology (AFIP) by Miettinen et al.
The complete guidelines are available on the NCCN website.
| Risk Stratification of Primary GIST by Mitotic Index, Size, and Site* | ||||||
|---|---|---|---|---|---|---|
|
Tumor Parameters
|
Risk of Progressive Disease* (%)
|
|||||
|
Group
|
Size
|
Mitotic rate |
Gastric
|
Jejunal/Ileum
|
Duodenal |
Rectum
|
|
1
|
≤ 2 cm** | ≤ 5 per 50 hpf | None (0%) | None (0%) | None (0%) | None (0%) |
|
2
|
>2 ≤ 5 cm | Very low (1.9%) | Low (4.3%) | Low (8.3%) | Low (8.5%) | |
|
3a
|
> 5 ≤ 10 cm | Low (3.6%) | Moderate (24%) | High (34%) ǂ | High† (57%) ǂ | |
|
3b
|
> 10 cm | Moderate (12%) | High (52%) | |||
|
|
||||||
|
4
|
≤ 2 cm | > 5 per 50 hpf | None† | High† (50%) | § | High (54%) |
|
5
|
> 2 cm ≤ 5 cm | Moderate (16%) | High (73%) | High (50%) | High (52%) | |
|
6a
|
> 5 cm ≤ 10 cm | High (55%) | High (85%) | High (86%) ǂ | High (71%) ǂ | |
|
6b
|
> 10 cm | High (86%) | High (90%) | |||
Abbreviations: GIST, gastrointestinal stromal tumor; hpf, high power field.
Adapted from Miettinen and Lasota, 2006. Data are based on long-term follow-up of 1055 gastric, 629 small intestinal,
144 duodenal, and 111 rectal GISTs. (Miettinen et al. 2001, 2005, and 2006).
*Defined as metastasis or tumor-related death.
†Denotes small numbers of cases.
ǂ Groups 3a and 3b or 6a and 6b are combined in duodenal and rectal GISTs because of small number of cases.
§ No tumors of such category were included in the study. Note that small intestinal and other intestinal GISTs show a markedly worse prognosis in many mitosis and size categories than gastric GISTs.
**Note: Only tumors <2 cm with mitotic rate <5 per 50 HPFs seem to remain consistently free of metastases in follow-up studies; all other categories involve metastatic risk (See table above). Small, ≤2 cm, mitotically active (>5 per 50 HPFs) GISTs in the rectum have >50% of metastatic rate. Such very small yet mitotically active GISTs most commonly occur in the rectum, where they can be found as palpable masses, whereas such tumors are extremely rare in the stomach and small intestine.
Image courtesy of Wikipedia; see Wikipedia articles for general information about: Duodenum / Jejunum / Ileum
GIST Nomogram
An easy to use tool is now available to calculate recurrence-free survival
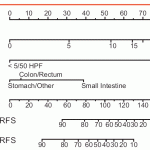
This nonogram, developed from 127 GIST patients treated at Memorial Sloan Kettering Cancer Center (MSKCC), can be used to predict the recurrence-free survival of GIST after surgery without receiving adjuvant therapy to help guide patient decision on selecting adjuvant imatinib therapy.
MSKCC has also developed an easy to use webpage where a patient enters the primary tumor location, tumor size, and checks a box if the mitotic rate is equal to or above 5 per 50 HPF. The prediction tool will then calculate the chances of remaining recurrence-free for 2 years and 5 years.
Caution: The MSKCC tool predicts recurrence-free survival (RFS). All of the other tools with the exception of the Joensuu GIST Risk Calculator on this page predict the risk of a recurrence. It is easy to confuse the two. In addition, it’s easy to confuse recurrence-free survival with survival. Survival is fairly straightforward – it’s a measure of whether you are alive or not.
Risk of recurrence is the predicted chance (given as a percentage) of your tumor coming back if you do not take imatinib. Recurrence free survival has nothing to do with how long you are going to live, instead it’s the percentage chance that you will remain both alive and without progression (i.e. your tumor has not come back or grown) after a given period of time. The nomogram estimates both the 2 year recurrence-free survival rate and the 5 year recurrence-free survival rate.
GIST Workshop Method
Especially useful when the primary tumor site is unknown
The following table is older (2002) and was developed from the Gastrointestinal Stromal Tumor (GIST) Workshop. It may still be particularly useful in cases of where the primary site is different than those listed in the table 1 or the primary site is unknown.
|
Proposed Approach for Defining Risk of Aggressive Behavior in GISTS
|
|||
|---|---|---|---|
| Size* | Mitotic Count+ | ||
| Very low risk | <2cm | <5/50 HPF | |
| Low risk | 2-5cm | <5/50 HPF | |
| Intermediate risk | >5cm | 6-10/50 HPF | |
| 5-10cm | <5/50 HPF | ||
| High risk | >5cm | >5/50 HPF | |
| >10cm | Any mitotic rate | ||
| Any size | >10/50 HPF | ||
| Abbreviation: HPF = high-power field. | |||
*Size represents the single largest dimension. Admittedly this may vary somewhat between prefixation and postfixation and between observers. There is a general but poorly defined sense that perhaps the size threshold for aggressive behavior should be 1 to 2 cm less in the small bowel than elsewhere.
+Ideally, mitotic count should be standardized according to surface area examined (based on size of high-powered fields), but there are no agreed-on definitions in this regard. Despite inevitable subjectivity in recognition of mitoses and variability in the area of high power fields, such mitotic counts still prove useful.
Note: The authors of this paper (Diagnosis of Gastrointestinal Stromal Tumors) also conclude that the risk categories as they define them in their paper “…should prove clinically useful, and in light of the uncertainties expressed herein and the well-recognized tendency of these troublesome tumors to pursue an indolent clinical course with a significant risk of late relapse, we also strongly advocate that all patients with GIST be carefully and regularly followed up for an indefinite period.”
GIST ROR
An interactive mobile tool developed by Novartis using various assessment methodologies
An interactive activity designed for use on an iPad, this program estimates the risk of recurrence in gastrointestinal stromal tumor (GIST) – using various assessment tools which were developed in conjunction with government institutions and major cancer organizations. The application features four hypothetical GIST patients and allows you to select input for key tumor-related prognostic factors. This easy-to-use application underscores not only the importance of assessing the risk of recurrence, but also hoe the estimated risk is influenced by various prognostic factors, as well as the selected assessment approach.
Caution: It is intended as an educational exercise for use by oncologists and other healthcare professionals who are involved in evaluation of patients after resection of primary GIST. Results are not intended for use in treatment decisions.
Click to download tool from iTunes
Click to download tool from Google Play
Future Directions
Gleevec is an outstanding drug and a lifeline for the majority of GIST patients. However, it does have side-effects and these can be significant for some patients. In addition, like all cancer treatments, it is expensive. Thus it is extremely desirable to find better methods to determine which patients need to take Gleevec and which patients are essentially “cured” by surgery and don’t need to take Gleevec.
In our February, 2012 newsletter, LRG Research Team member, Dr. Maria Debiec-Rychter, describes three new genomic-based methods that appear to significantly outperform current methods of risk assessment:
- CINSARC
- AURKA expression
- Genomic Index
One of these methods, the CINSARC method, appears to have broader applicability to the wider sarcoma community as well. Importantly, the DNA CGHarray technique performed by Dr. Debiec-Rychter and colleagues can be performed from paraffin-embedded tumor tissue, the type of tissue that is already available/existing for most GIST patients.
Next Steps:
Where can I find factors to calculate rate of recurrence (ROR)? (tumor size, mitotic index, location of tumor)
Treatments for GIST
Newly Diagnosed Patients
References
- Diagnosis of Gastrointestinal Stromal Tumors: A Consensus Approach-Christopher D. M. Fletcher, MD, FRCPATH, Jules J. Berman, MD, PhD, Christopher Corless, MD, PhD, Fred Gorstein, MD, Jerzy Lasota, MD, PhD, B. Jack Longley, MD, Markku Miettinen, MD, Timothy J. O’Leary, MD, PhD, Helen Remotti, MD, Brian P. Rubin, MD, Phd, Barry Shmookler, MD, Leslie H. Sobin, MD, and Sharon W. Weiss, MD.
- Prognostic value of KIT/PDGFRA mutations in gastrointestinal stromal tumours (GIST): Polish Clinical GIST Registry experience.Wozniak A, Rutkowski P, Piskorz A, Ciwoniuk M, Osuch C, Bylina E, Sygut J, Chosia M, Rys J, Urbanczyk K, Kruszewski W, Sowa P, Siedlecki J, Debiec-Rychter M, Limon J; on behalf of Polish Clinical GIST Registry. Source Department of Biology and Genetics, Medical University of Gdansk, Gdansk, Poland.
- Development and validation of a prognostic nomogram for recurrence-free survival after complete surgical resection of localised primary gastrointestinal stromal tumour: a retrospective analysis. Gold JS, Gönen M, Gutiérrez A, Broto JM, García-del-Muro X, Smyrk TC, Maki RG, Singer S, Brennan MF, Antonescu CR, Donohue JH, DeMatteo RP.
- NCCN Task Force Report: Update on the Management of Patients with Gastrointestinal Stromal Tumors George D. Demetri, MD, Margaret von Mehren, MD, Cristina R. Antonescu, MD, Ronald P. DeMatteo, MD, Kristen N. Ganjoo, MD, Robert G. Maki, MD, PhD, Peter W.T. Pisters, MD, Chandrajit P. Raut, MD, MSc, Richard F. Riedel, MD, Scott Schuetze, MD, PhD, Hema M. Sundar, PhD, Jonathan C. Trent, MD, PhD and Jeffrey D. Wayne, MD.
- Gastrointestinal Stromal Tumors of the Stomach. A Clinicopathologic, Immunohistochemical, and Molecular Genetic Study of 1765 Cases With Long-term Follow-up. American Journal of Surgical Pathology. 29(1):52-68, January 2005. Miettinen, Markku MD; Sobin, Leslie H MD +; Lasota, Jerzy MD.
- Gastrointestinal stromal tumors: pathology and prognosis at different sites. Miettine, M., Lasota J.
- Gastrointestinal stromal tumors, intramural leiomyomas, and leiomyosarcomas in the rectum and anus: a clinicopathologic, immunohistochemical, and molecular genetic study of 144 cases. Miettinen M, Furlong M, Sarlomo-Rikala M, Burke A, Sobin LH, Lasota J.
- ASCO 2010-Relation of tumor pathologic and molecular features to outcome after surgical resection of localized primary gastrointestinal stromal tumor (GIST): Results of the intergroup phase III trial ACOSOG Z9001. C. L. Corless, K. V. Ballman, C. Antonescu, C. D. Blanke, M. E. Blackstein, G. D. Demetri, M. von Mehren, R. G. Maki, P. W. Pisters, R. P. DeMatteo, American College of Surgeons Oncology Group; Portland VA Medical Center and Oregon Health & Science University Knight Cancer Institute, Portland, OR; Mayo Clinic Rochester, Rochester, MN; Memorial Sloan-Kettering Cancer Center, New York, NY; University of British Columbia/British Columbia Cancer Agency, Vancouver, BC, Canada; Mount Sinai Hospital, Toronto, ON, Canada; Ludwig Center, Dana-Farber Cancer Institute/Harvard Cancer Center and Sarcoma Center, Boston, MA; Fox Chase Cancer Center, Philadelphia, PA; University of Texas M. D. Anderson Cancer Center, Houston, TX.
- The Novel Marker, DOG1, Is Expressed Ubiquitously in Gastrointestinal Stromal Tumors Irrespective of KIT or PDGFRA Mutation Status West RB, Corless CL, Chen X, Rubin BP, Subramanian S, Montgomery K, Zhu S, Ball CA, Nielsen TO, Patel R, Goldblum JR, Brown PO, Heinrich MC, van de Rijn M.
- Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts Prof Heikki Joensuu MD,Aki Vehtari DSci,Jaakko Riihimäki MSc,Toshirou Nishida MD,Sonja E Steigen MD,Peter Brabec MSci,Prof Lukas Plank MD,Bengt Nilsson MD,Claudia Cirilli BSc,Chiara Braconi MD,Andrea Bordoni MD,Magnus K Magnusson MD,Zdenek Linke MD,Jozef Sufliarsky MD,Massimo Federico MD,Jon G Jonasson MD,Angelo Paolo Dei Tos MD,Piotr Rutkowski MD.
- Mitotic Checkpoints and Chromosome Instability are Strong Predictors of Clinical Outcome in Gastrointestinal Stromal Tumors Pauline Lagarde, Gaëlle Pérot, Audrey Kauffmann, Céline Brulard, Valérie Dapremont, Isabelle Hostein, Agnès Neuville, Agnieszka Wozniak, Raf Sciot, Patrick Schöffski, Alain Aurias, Jean-Michel Coindre, Maria Debiec-Rychter, Frédéric Chibon.
- Risk stratification of patients diagnosed with gastrointestinal stromal tumor Heikki Joensuu MD, PhD.