State of the GIST Community Part 3: What do we know about mutational testing?
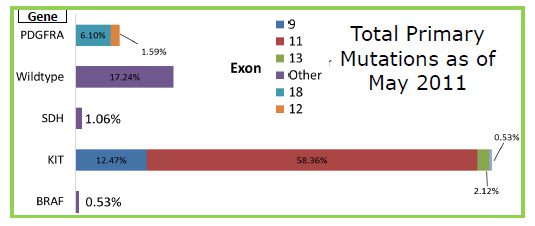
As of May 1, 2011, the LRG patient registry only received 377 reports of mutations out of 1,327 patients, which only represents 28 percent of the entire registry. Part of this may be related to the fact that mutational testing is not common practice at diagnosis.