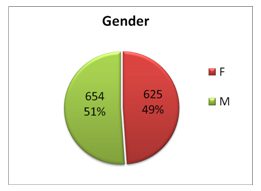
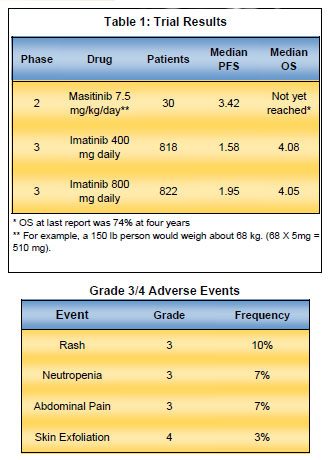
Breaking News Update: TDM renews plasma testing
On April 21, 2010, the U.S. Food and Drug Administration (FDA) issued a warning letter to Novartis criticizing its promotion of blood level testing on its CML and GIST Alliance websites. As of September 30, 2010, Novartis terminated the CML and GIST Alliance programs which it had contracted with TDM Pharmaceutical Research, LLC. Since then, the Life Raft Group has been working tirelessly behind the scenes to try and resume this critical testing procedure as it has important potential for managing GIST patient care.