State of the GIST community: Life Raft Group’s patient registry demographics
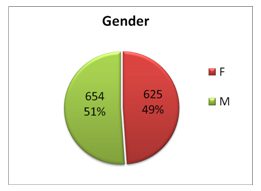
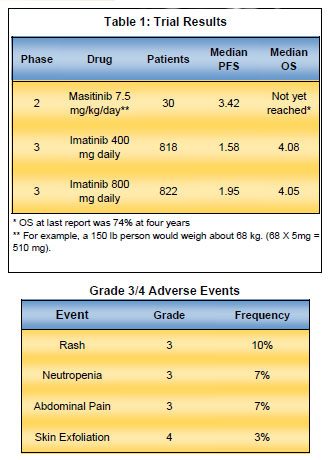
As of December 31, 2010 the Life Raft Group’s Patient Registry has now accounted for 1,279 cases of GIST. The members of this registry have reported their clinical information to us as it pertains to their treatment of GIST. Of these 1,279 cases, we are here to report some key demographics that will help you understand who is a part of Life Raft Group GIST Community.