Genetic Markers of Progression in GISTs and Their Significance
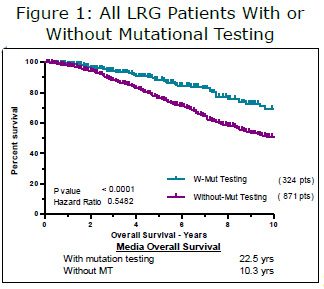
Today there are four major methods of determining risk of recurrence after initial surgery. These methods are pretty good at identifying low-risk and highrisk tumors. However, there is a fairly large group that fall between low-risk and high-risk. Those with so-called “moderate” or “intermediate” risk tumors are faced with the decision of whether or not to take Gleevec. New guidelines exist that recommend taking Gleevec for at least three years, with many experts advocating longer periods for high-risk patients.